Lab Anim Res.
2013 Sep;29(3):156-161. 10.5625/lar.2013.29.3.156.
Focal cerebral ischemic injury decreases calbindin expression in brain tissue and HT22 cells
- Affiliations
-
- 1Department of Anatomy, College of Veterinary Medicine, Research Institute of Life Science, Gyeongsang National University, Jinju, Korea. pokoh@gnu.ac.kr
- KMID: 2312108
- DOI: http://doi.org/10.5625/lar.2013.29.3.156
Abstract
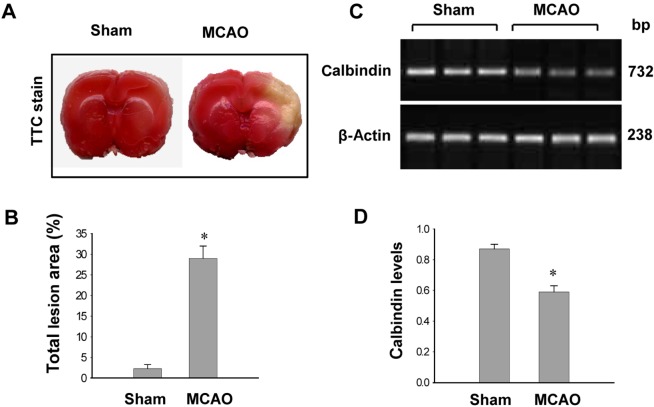
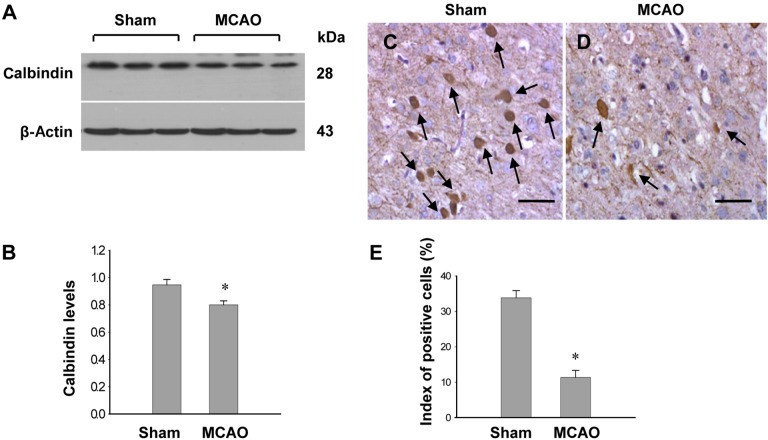
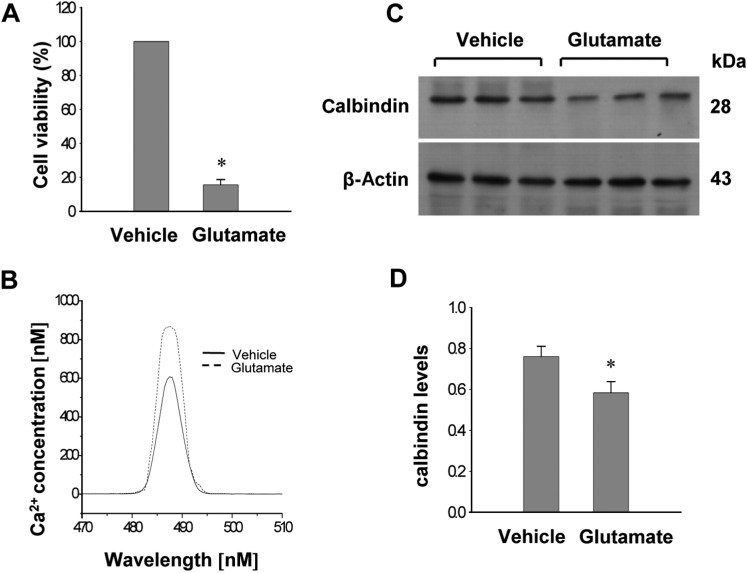
- Calbindin is a calcium binding protein that controls intracellular calcium levels and has a neuroprotective function against apoptotic stimuli. We investigated the expression of calbindin in ischemic brain injury. Focal cerebral ischemia was induced in male rats by middle cerebral artery occlusion (MCAO) and cerebral cortices were collected 24 h after MCAO. Cerebral ischemia significantly increased infarct volume. RT-PCR and Western blot analyses showed that MCAO injury induced a decrease of calbindin expression. Moreover, immunohistochemical staining showed that the number of calbindin-positive cells decreased in ischemic regions of MCAO-operated animals. In cultured hippocampal-derived cell lines, glutamate exposure increased intracellular Ca2+ concentrations and decreased calbindin expression. Taken together, both in vivo and in vitro results demonstrated decreases of calbindin after neuronal cell injury. These results suggest that decreases of calbindin in ischemic brain injury contribute to neuronal cell death.
MeSH Terms
-
Animals
Blotting, Western
Brain
Brain Injuries
Brain Ischemia
Calcium
Calcium-Binding Protein, Vitamin D-Dependent
Carrier Proteins
Cell Death
Cell Line
Cerebral Cortex
Glutamic Acid
Humans
Infarction, Middle Cerebral Artery
Male
Neurons
Rats
Calcium
Calcium-Binding Protein, Vitamin D-Dependent
Carrier Proteins
Glutamic Acid
Figure
Reference
-
1. Sims NR, Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophys Acta. 2010; 1802(1):80–91. PMID: 19751827.
Article2. Flynn RW, MacWalter RS, Doney AS. The cost of cerebral ischaemia. Neuropharmacology. 2008; 55(3):250–256. PMID: 18573263.
Article3. Starkov AA, Chinopoulos C, Fiskum G. Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium. 2004; 36(3-4):257–264. PMID: 15261481.
Article4. Orrenius S, Nicotera P. The calcium ion and cell death. J Neural Transm Suppl. 1994; 43:1–11. PMID: 7884392.5. Kristián T, Siesjö BK. Calcium in ischemic cell death. Stroke. 1998; 29(3):705–718. PMID: 9506616.
Article6. Bano D, Nicotera P. Ca2+ signals and neuronal death in brain ischemia. Stroke. 2007; 38(2):674–676. PMID: 17261713.7. Kanai Y, Bhide PG, DiFiglia M, Hediger MA. Neuronal high-affinity glutamate transport in the rat central nervous system. Neuroreport. 1995; 6(17):2357–2362. PMID: 8747153.
Article8. Penugonda S, Mare S, Lutz P, Banks WA, Ercal N. Potentiation of lead-induced cell death in PC12 cells by glutamate: protection by N-acetylcysteine amide (NACA), a novel thiol antioxidant. Toxicol Appl Pharmacol. 2006; 216(2):197–205. PMID: 16781745.
Article9. Hazell AS, Itzhak Y, Liu H, Norenberg MD. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) decreases glutamate uptake in cultured astrocytes. J Neurochem. 1997; 68(5):2216–2219. PMID: 9109551.
Article10. Manev H, Favaron M, Guidotti A, Costa E. Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol Pharmacol. 1989; 36(1):106–112. PMID: 2568579.11. Heizmann CW, Braun K. Changes in Ca(2+)-binding proteins in human neurodegenerative disorders. Trends Neurosci. 1992; 15(7):259–264. PMID: 1381122.
Article12. Baimbridge KG, Celio MR, Rogers JH. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992; 15(8):303–308. PMID: 1384200.
Article13. Bastianelli E. Distribution of calcium-binding proteins in the cerebellum. Cerebellum. 2003; 2(4):242–262. PMID: 14964684.
Article14. Christakos S, Barletta F, Huening M, Dhawan P, Liu Y, Porta A, Peng X. Vitamin D target proteins: function and regulation. J Cell Biochem. 2003; 88(2):238–244. PMID: 12520521.
Article15. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989; 20(1):84–91. PMID: 2643202.
Article16. Sung JH, Kim MO, Koh PO. Ferulic acid attenuates the focal cerebral ischemic injury-induced decrease in parvalbumin expression. Neurosci Lett. 2012; 516(1):146–150. PMID: 22490888.
Article17. Koh PO. Ferulic acid attenuates the injury-induced decrease of protein phosphatase 2A subunit B in ischemic brain injury. PLoS One. 2013; 8(1):e54217. PMID: 23349830.
Article18. Maher P, Davis JB. The role of monoamine metabolism in oxidative glutamate toxicity. J Neurosci. 1996; 16(20):6394–6401. PMID: 8815918.
Article19. Tan S, Sagara Y, Liu Y, Maher P, Schubert D. The regulation of reactive oxygen species production during programmed cell death. J Cell Biol. 1998; 141(6):1423–1432. PMID: 9628898.
Article20. Malgaroli A, Milani D, Meldolesi J, Pozzan T. Fura-2 measurement of cytosolic free Ca2+ in monolayers and suspensions of various types of animal cells. J Cell Biol. 1987; 105(5):2145–2155. PMID: 3680375.
Article21. Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985; 260(6):3440–3450. PMID: 3838314.
Article22. Guo Q, Christakos S, Robinson N, Mattson MP. Calbindin D28k blocks the proapoptotic actions of mutant presenilin 1: reduced oxidative stress and preserved mitochondrial function. Proc Natl Acad Sci U S A. 1998; 95(6):3227–3232. PMID: 9501245.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Focal Cerebral Ischemia Induces Decrease of Astrocytic Phosphoprotein PEA-15 in Brain Tissue and HT22 Cells
- Effects of Experimental Focal Ischemia of PV- and Calbindin-Immunoreactive Neuron of Rat Neocortex
- Cerebral ischemic injury decreases α-synuclein expression in brain tissue and glutamate-exposed HT22 cells
- Ischemic brain injury decreases dynamin-like protein 1 expression in a middle cerebral artery occlusion animal model and glutamate-exposed HT22 cells
- Focal Cerebral Ischemia Reduces Protein Phosphatase 2A Subunit B Expression in Brain Tissue and HT22 Cells