Lab Anim Res.
2011 Mar;27(1):73-76. 10.5625/lar.2011.27.1.73.
Focal Cerebral Ischemia Reduces Protein Phosphatase 2A Subunit B Expression in Brain Tissue and HT22 Cells
- Affiliations
-
- 1Department of Anatomy, College of Veterinary Medicine, Research Institute of Life Sciences, Gyeongsang National University, Jinju, Republic of Korea. pokoh@gnu.ac.kr
- KMID: 2391863
- DOI: http://doi.org/10.5625/lar.2011.27.1.73
Abstract
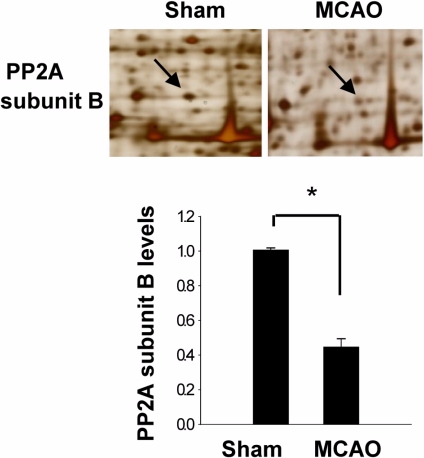
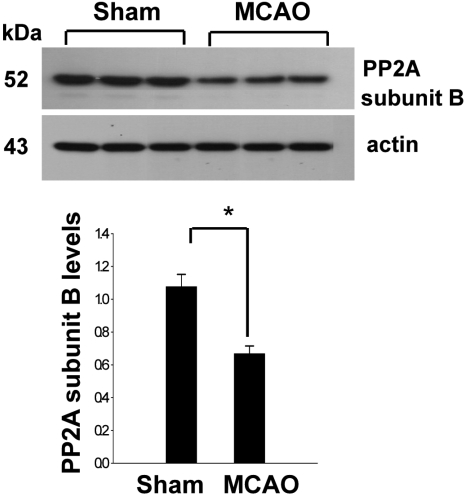
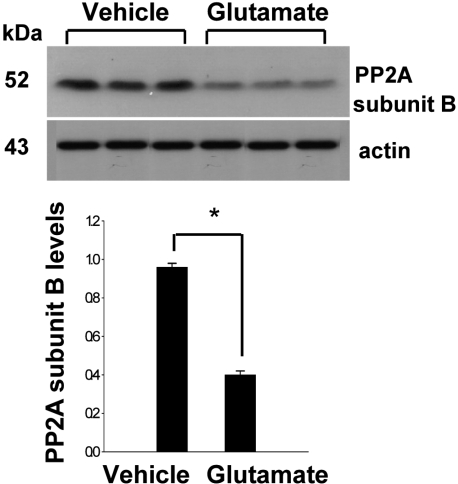
- Protein phosphatase 2A (PP2A) is a serine and threonine protein phosphatase that regulates cell cycle progression and apoptosis. PP2A is composed of various subunits. Among these subunits, subunit B plays an important role in the modulation of PP2A function in the brain. This study investigated PP2A subunit B expression levels after neuronal cell injury. Middle cerebral artery occlusions (MCAO) were surgically induced in adult male rats to induce focal cerebral ischemic injury, and brain tissues were collected 24 h after MCAO. A proteomic approach revealed reduction of PP2A subunit B protein spots in MCAO-operated animals in comparison to sham-operated animals. Western blot analysis confirmed that MCAO induces reductions in PP2A subunit B levels. Moreover, glutamate exposure induces neuronal cell death and leads to reductions of PP2A subunit B levels in a hippocampal-derived cell line. This study demonstrated the decrease of PP2A subunit B in ischemic neuronal cell injury. These results suggest that the decrease of PP2A subunit B after ischemic brain injury can mediate neuronal cell death.
MeSH Terms
Figure
Cited by 1 articles
-
Curcumin treatment recovery the decrease of protein phosphatase 2A subunit B induced by focal cerebral ischemia in Sprague-Dawley rats
Fawad-Ali Shah, Dong-Ju Park, Sang-Ah Gim, Phil-Ok Koh
Lab Anim Res. 2015;31(3):134-138. doi: 10.5625/lar.2015.31.3.134.
Reference
-
1. Ferrer I, Planas AM. Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle inthe penumbra. J Neuropathol Exp Neurol. 2003; 62(4):329–339. PMID: 12722825.2. Gong CK, Lidsky T, Wegiel J, Zuck L, Grundke-Iqbal I, Iqbal K. Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain. Implications for neurofibrillary degeneration in Alzheimer's disease. J Biol Chem. 2000; 275(8):5535–5544. PMID: 10681533.3. Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001; 353(Pt 3):417–439. PMID: 11171037.
Article4. Koh PO. 17Beta-estradiol prevents the glutamate-induced decrease of Akt and its downstream targets in HT22 cells. J Vet Med Sci. 2007; 69(3):285–288. PMID: 17409645.
Article5. Koh PO. Proteomic analysis of focal cerebral ischemic injury in male rats. J Vet Med Sci. 2010; 72(2):181–185. PMID: 19942814.
Article6. Li Y, Chopp M, Powers C, Jiang N. Apoptosis and protein expression after focal cerebral ischemia in rat. Brain Res. 1997; 765(2):301–312. PMID: 9313903.
Article7. Liu R, Wang JZ. Protein phosphatase 2A in Alzheimer's disease. Pathophysiology. 2009; 16(4):273–277. PMID: 19278841.
Article8. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989; 20(1):84–91. PMID: 2643202.
Article9. Maher P, Davis JB. The role of monoamine metabolism inoxidative glutamate toxicity. J Neurosci. 1996; 16(20):6394–6401. PMID: 8815918.10. Millward TA, Zolnierowicz S, Hemmings BA. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem Sci. 1999; 24(5):186–191. PMID: 10322434.
Article11. Siesjö BK. Mechanisms of ischemic brain damage. Crit Care Med. 1988; 16(10):954–963. PMID: 3048896.12. Sontag E, Nunbhakdi-Craig V, Lee G, Bloom GS, Mumby MC. Regulation of the phosphorylation state and microtubule-binding activity of Tau by protein phosphatase 2A. Neuron. 1996; 17(6):1201–1207. PMID: 8982166.
Article13. Sontag E. Protein phosphatase 2A: the Trojan Horse of cellular signaling. Cell Signal. 2001; 13(1):7–16. PMID: 11257442.14. Stone SR, Hofsteenge J, Hemmings BA. Molecular cloning of cDNAs encoding two isoforms of the catalytic subunit ofprotein phosphatase 2A. Biochemistry. 1987; 26(23):7215–7220. PMID: 2827745.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Curcumin treatment recovery the decrease of protein phosphatase 2A subunit B induced by focal cerebral ischemia in Sprague-Dawley rats
- Epigallocatechin gallate restores the reduction of protein phosphatase 2 A subunit B caused by middle cerebral artery occlusion
- Focal Cerebral Ischemia Induces Decrease of Astrocytic Phosphoprotein PEA-15 in Brain Tissue and HT22 Cells
- Decrease of protein phosphatase 2A subunit B by glutamate exposure in the cerebral cortex of neonatal rats
- Chlorogenic acid regulates the expression of protein phosphatase 2A subunit B in the cerebral cortex of a rat stroke model and glutamate-exposed neurons