Lab Anim Res.
2013 Mar;29(1):19-26. 10.5625/lar.2013.29.1.19.
Age-associated changes in pancreatic exocrine secretion of the isolated perfused rat pancreas
- Affiliations
-
- 1Teaching Center of Functional Experimental Science, College of Basic Medicine, Yanbian University, Yanji, China.
- 2Department of Veterinary Medicine, College of Agriculture, Yanbian University, Yanji, China.
- 3Department of Physiology, and Institute of Neurodegeneration and Neuroregeneration, College of Medicine, Hallym University, Chuncheon, Korea. yylee@hallym.ac.kr
- 4Department of Occupational Therapy, Dongnam Health College, Suwon, Korea.
- 5Department of Oriental Medicine Materials, Dongshin University, Naju, Korea. dhj1221@dongshin.ac.kr
- 6Department of Neurobiology, School of Medicine, Kangwon National University, Chuncheon, Korea.
- KMID: 2312099
- DOI: http://doi.org/10.5625/lar.2013.29.1.19
Abstract
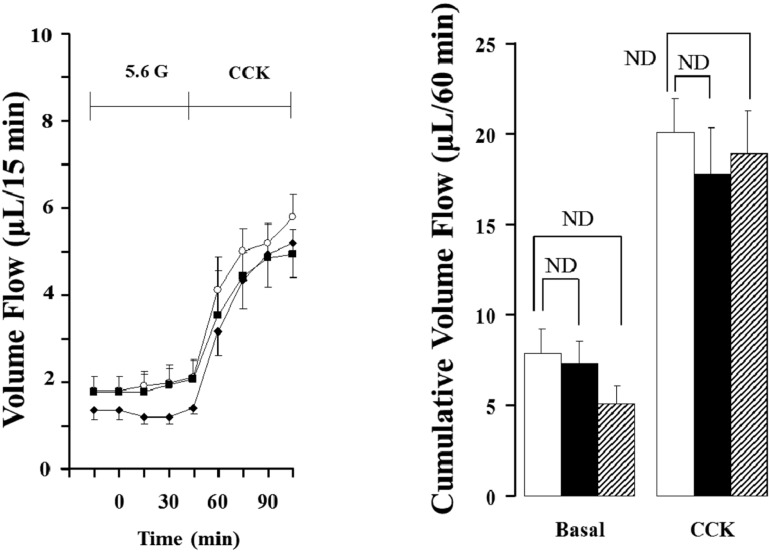
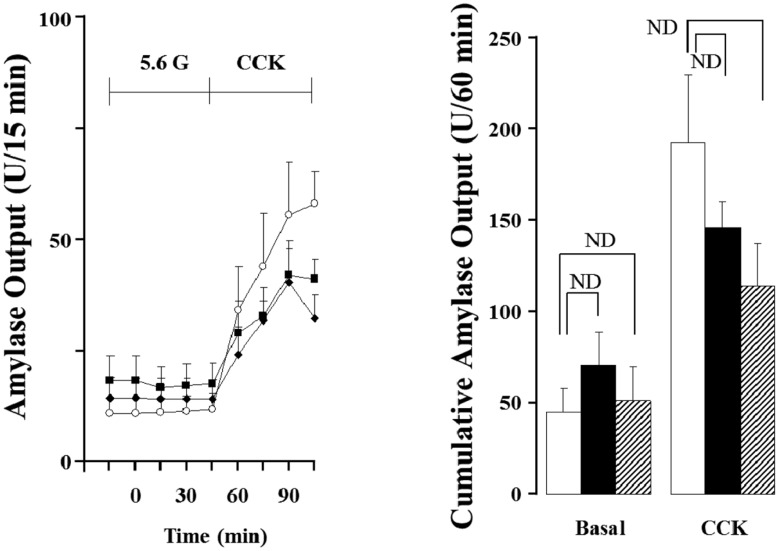
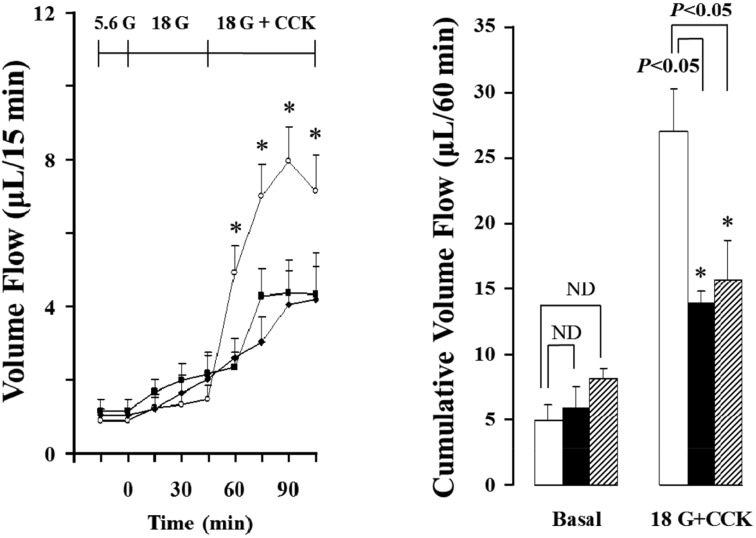
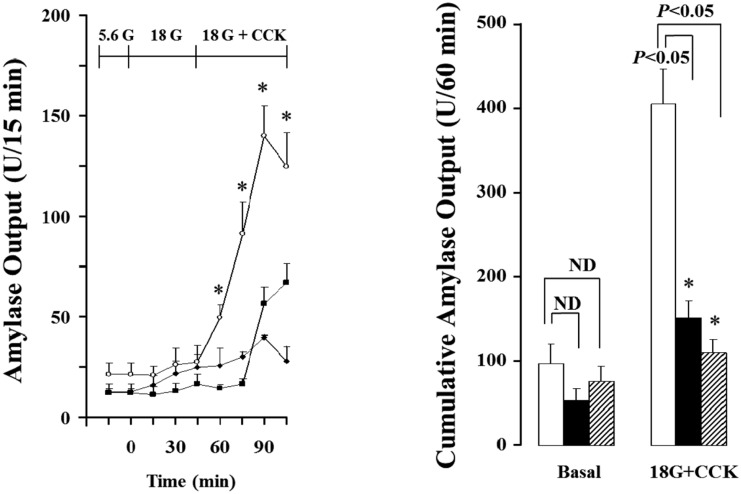
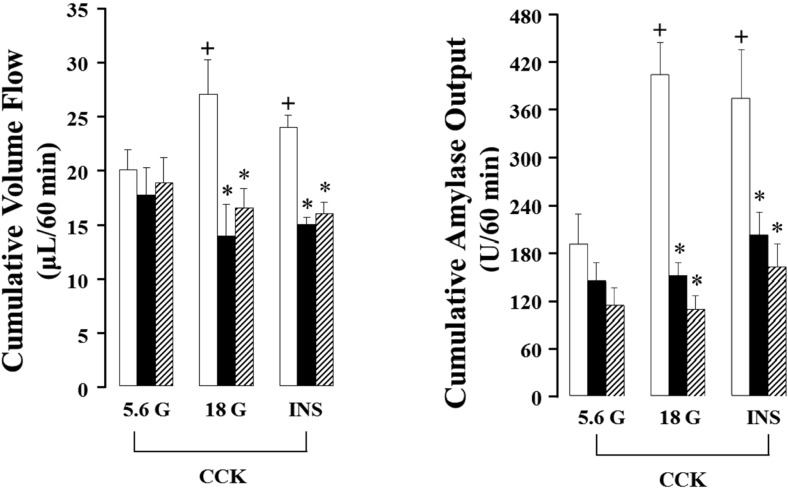
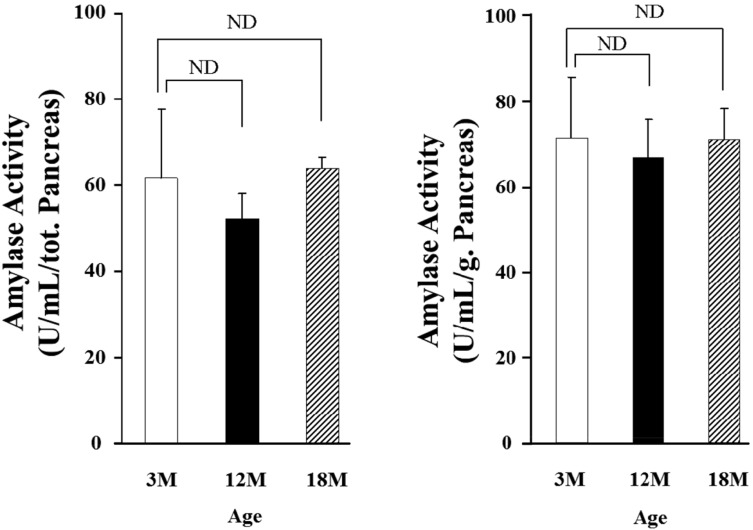
- Gut functions, such as gastrointestinal motility, gastric secretion and pancreatic secretion, were reduced with age. Glucose tolerance is impaired, and the release of insulin and beta-cell's sensitivity on glucose are reduced with age. However, a lot of controversial data have been reported as insulin concentrations after glucose ingestion are either higher or no different in elderly and young subjects. Thus, this study was aimed to investigate whether aging could affect pancreatic exocrine secretion and its action mechanisms. An isolated perfused rat pancreatic model was used to exclude the effects of external nerves or hormones. Pancreatic secretion was increased by CCK under 5.6 mM glucose background in the isolated perfused pancreas of young (3 months), 12 months and 18 months aged rats. There was no significant difference between young and aged rats. In 3 months old rats, CCK-stimulated pancreatic secretion was potentiated under 18 mM glucose background. However, the potentiation effects of endogenous insulin and CCK were not observed in 12 and 18 months old rats. Exogenous insulin also potentiated CCK-stimulated pancreatic secretion in 3 months old rats. Similarly, exogenous insulin failed to potentiate CCK-stimulated pancreatic secretion as that of 3 months old rats. Wet weight of pancreas and amylase content in pancreatic tissue were not changed with age. These results indicate that pancreatic exocrine secretion is reduced with age and endogenous insulin secretion and/or action is involved in this phenomenon.
Keyword
MeSH Terms
Figure
Reference
-
1. Werner I, Hambraeus L. The digestive capacity of elderly people. The effect of a high protein diet. Acta Soc Med Ups. 1971; 76(5-6):239–242. PMID: 5141080.2. Sonnenberg A, Koch TR. Physician visits in the United States for constipation: 1958 to 1986. Dig Dis Sci. 1989; 34(4):606–611. PMID: 2784759.
Article3. Lee YL, Kwon HY, Park HS, Lee TH, Park HJ. The role of insulin in the interaction of secretin and cholecystokinin in exocrine secretion of the isolated perfused rat pancreas. Pancreas. 1996; 12(1):58–63. PMID: 8927620.
Article4. Bergeron JJ, Rachubinski R, Searle N, Sikstrom R, Borts D, Bastian P, Posner BI. Radioautographic visualization of in vivo insulin binding to the exocrine pancreas. Endocrinology. 1980; 107(4):1069–1080. PMID: 6997018.
Article5. Sankaran H, Iwamoto Y, Korc M, Williams JA, Goldfine ID. Insulin action in pancreatic acini from streptozotocin-treated rats. II. Binding of 125I-insulin to receptors. Am J Physiol. 1981; 240(1):G63–G68. PMID: 7006418.
Article6. Sjodin L, Holmberg K, Lyden A. Insulin receptors on pancreatic acinar cells in guinea pigs. Endocrinology. 1984; 115(3):1102–1109. PMID: 6086285.
Article7. Goldfine ID, Kriz BM, Wong KY, Hradek G, Jones AL, Williams JA. Insulin action in pancreatic acini from streptozotocin-treated rats. III. Electron microscope autoradiography of 125I-insulin. Am J Physiol. 1981; 240(1):G69–G75. PMID: 7006419.
Article8. Curry DL, Reaven G, Reaven E. Glucose-induced insulin secretion by perfused pancreas of 2- and 12-mo-old Fischer 344 rats. Am J Physiol. 1984; 247(3 Pt 1):E385–E388. PMID: 6383072.
Article9. Aizawa T, Komatsu M, Sato Y, Ishihara F, Suzuki N, Nishii N, Hashizume K, Yamada T. Insulin secretion by the pancreatic beta cell of aged rats. Pancreas. 1994; 9(4):454–459. PMID: 7937694.10. Elahi D, Muller DC, Andersen DK, Tobin JD, Andres R. The effect of age and glucose concentration on insulin secretion by the isolated perfused rat pancreas. Endocrinology. 1985; 116(1):11–16. PMID: 3880537.
Article11. Morgan ZR, Feldman M. The liver, biliary tract and pancreas in the aged: an anatomic and laboratory evaluation. J Am Geriatr Soc. 1957; 5(1):59–65. PMID: 13405716.
Article12. Ammann R, Sulser H. ["Senile" chronic pancreatitis; a new nosologic entity? Studies in 38 cases. Indications of a vascular origin and relationship to the primarily painless chronic pancreatitis]. Schweiz Med Wochenschr. 1976; 106(13):429–437. PMID: 772803.13. Khalil T, Fujimura M, Townsend CM Jr, Greeley GH Jr, Thompson JC. Effect of aging on pancreatic secretion in rats. Am J Surg. 1985; 149(1):120–125. PMID: 3966629.
Article14. Park HJ, Lee YL, Kwon HY. Effects of pancreatic polypeptide on insulin action in exocrine secretion of isolated rat pancreas. J Physiol. 1993; 463:421–429. PMID: 7504106.
Article15. Kanno T, Saito A. The potentiating influences of insulin on pancreozymin-induced hyperpolarization and amylase release in the pancreatic acinar cell. J Physiol. 1976; 261(3):505–521. PMID: 978585.
Article16. Jiang ZE, Shin BN, Kim IH, Lee HJ, Yong JH, Lee MJ, Won MH, Lee YL. Roles of Non-cholinergic Intrapancreatic Nerves, Serotonergic Nerves, on Pancreatic Exocrine Secretion in the Isolated Perfused Rat Pancreas. Korean J Physiol Pharmacol. 2011; 15(5):307–312. PMID: 22128264.
Article17. Garry DJ, Garry MG, Williams JA, Mahoney WC, Sorenson RL. Effects of islet hormones on amylase secretion and localization of somatostatin binding sites. Am J Physiol. 1989; 256(5 Pt 1):G897–G904. PMID: 2470260.
Article18. Saito A, Williams JA, Kanno T. Potentiation of cholecystokinin-induced exocrine secretion by both exogenous and endogenous insulin in isolated and perfused rat pancreata. J Clin Invest. 1980; 65(4):777–782. PMID: 6987265.
Article19. Lee KY, Zhou L, Ren XS, Chang TM, Chey WY. An important role of endogenous insulin on exocrine pancreatic secretion in rats. Am J Physiol. 1990; 258(2 Pt 1):G268–G274. PMID: 1689549.
Article20. Reaven E, Curry D, Moore J, Reaven G. Effect of age and environmental factors on insulin release from the perfused pancreas of the rat. J Clin Invest. 1983; 71(2):345–350. PMID: 6337185.
Article21. Basu R, Breda E, Oberg AL, Powell CC, Dalla Man C, Basu A, Vittone JL, Klee GG, Arora P, Jensen MD, Toffolo G, Cobelli C, Rizza RA. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003; 52(7):1738–1748. PMID: 12829641.22. Scheen AJ, Sturis J, Polonsky KS, Van Cauter E. Alterations in the ultradian oscillations of insulin secretion and plasma glucose in aging. Diabetologia. 1996; 39(5):564–572. PMID: 8739916.
Article23. Borg LA, Dahl N, Swenne I. Age-dependent differences in insulin secretion and intracellular handling of insulin in isolated pancreatic islets of the rat. Diabete Metab. 1995; 21(6):408–414. PMID: 8593921.24. Lipson LG, Bush MJ, Tietjen GE, Yoon A. Role of the adenylate cyclase system in altered insulin release from islets of Langerhans of aging rats. Acta Endocrinol (Copenh). 1981; 96(2):222–226. PMID: 6258370.
Article25. Draznin B, Steinberg JP, Leitner JW, Sussman KE. The nature of insulin secretory defect in aging rats. Diabetes. 1985; 34(11):1168–1173. PMID: 2412920.
Article26. Burch PT, Berner DK, Leontire A, Vogin A, Matschinsky BM, Matschinsky FM. Metabolic adaption of pancreatic islet tissue in aging rats. J Gerontol. 1984; 39(1):2–6. PMID: 6228572.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Roles of Gonadal Steroids on Exocrine Secretion of Isolated Perfused Rat Pancreas
- A role of endogenous somatostatin in exocrine secretion induced by intrapancreatic cholinergic activation
- Mechanism of action of pancreatic polypeptide (PP) on pancreatic exocrine secretion in isolated rat pancreas
- Effects of gamma-Aminobutyric Acid on Intrinsic Cholinergic Action in Exocrine Secretion of Isolated, Perfused Rat Pancreas
- Exogenous cysteamine increases basal pancreatic exocrine secretion in the rat