Lab Anim Res.
2010 Jun;26(2):181-196. 10.5625/lar.2010.26.2.181.
Safety Evaluation of Human Fibroblasts in Mice: Tumorigenicity, 13-week Toxicity and Distribution Studies
- Affiliations
-
- 1Department of Experimental Animal Research, Clinical Research Institute, Seoul National University Hospital, Seoul, Korea. bckang@snu.ac.kr
- 2Department of Immunology and Laboratory Animal Medicine, College of Medicine, Seoul National University, Seoul, Korea.
- 3S.BIOMEDICS Co., Ltd. Seoul, Korea.
- KMID: 2312064
- DOI: http://doi.org/10.5625/lar.2010.26.2.181
Abstract
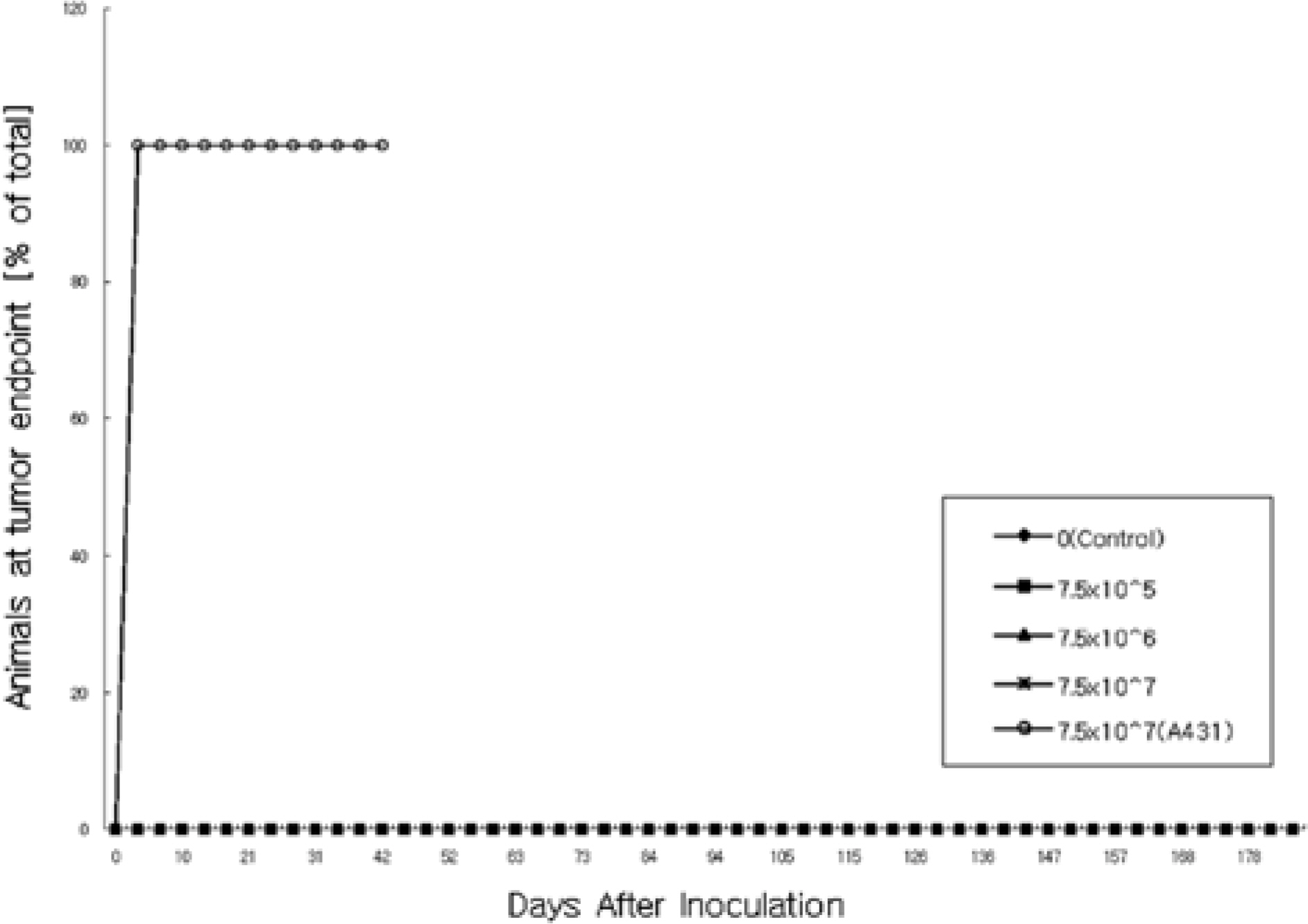
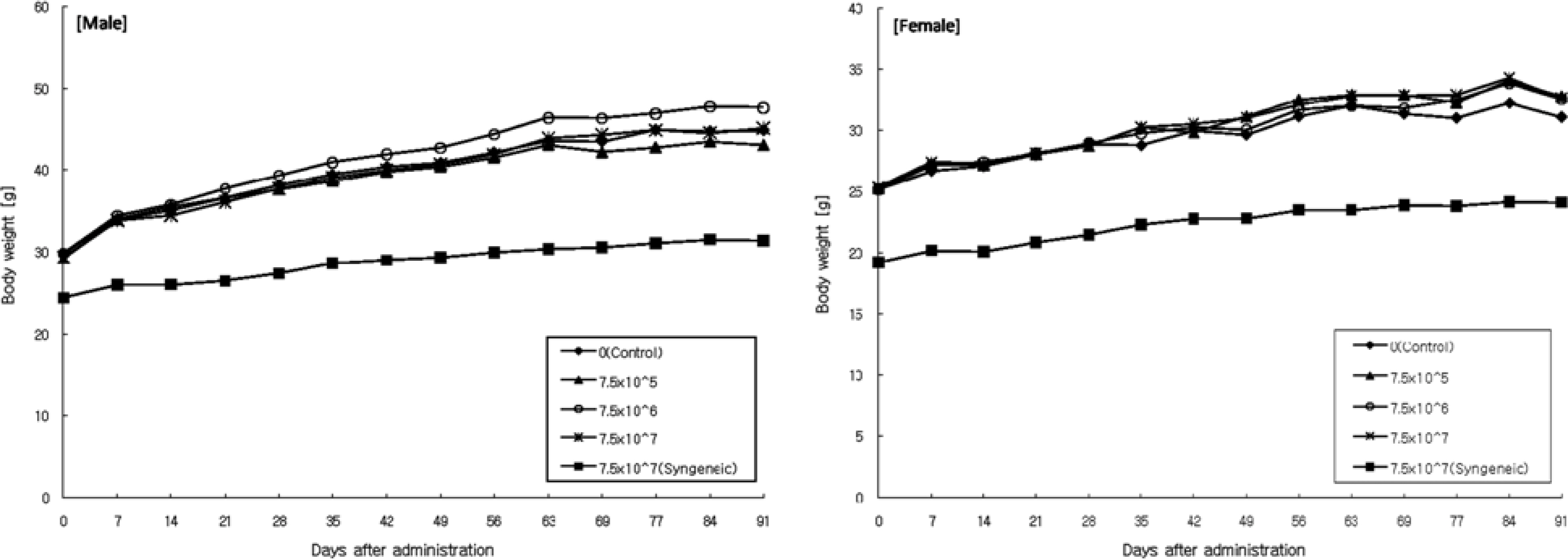
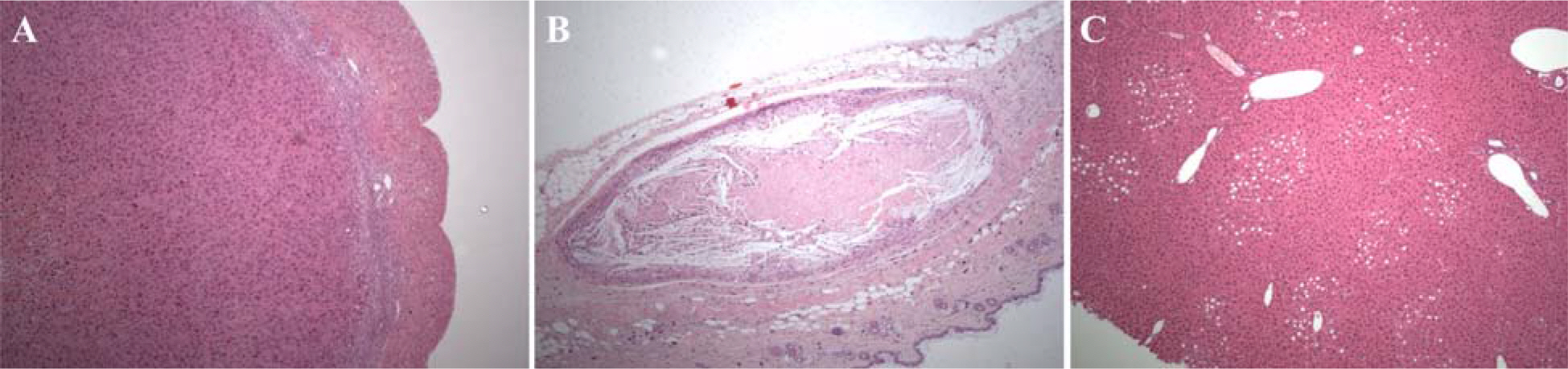
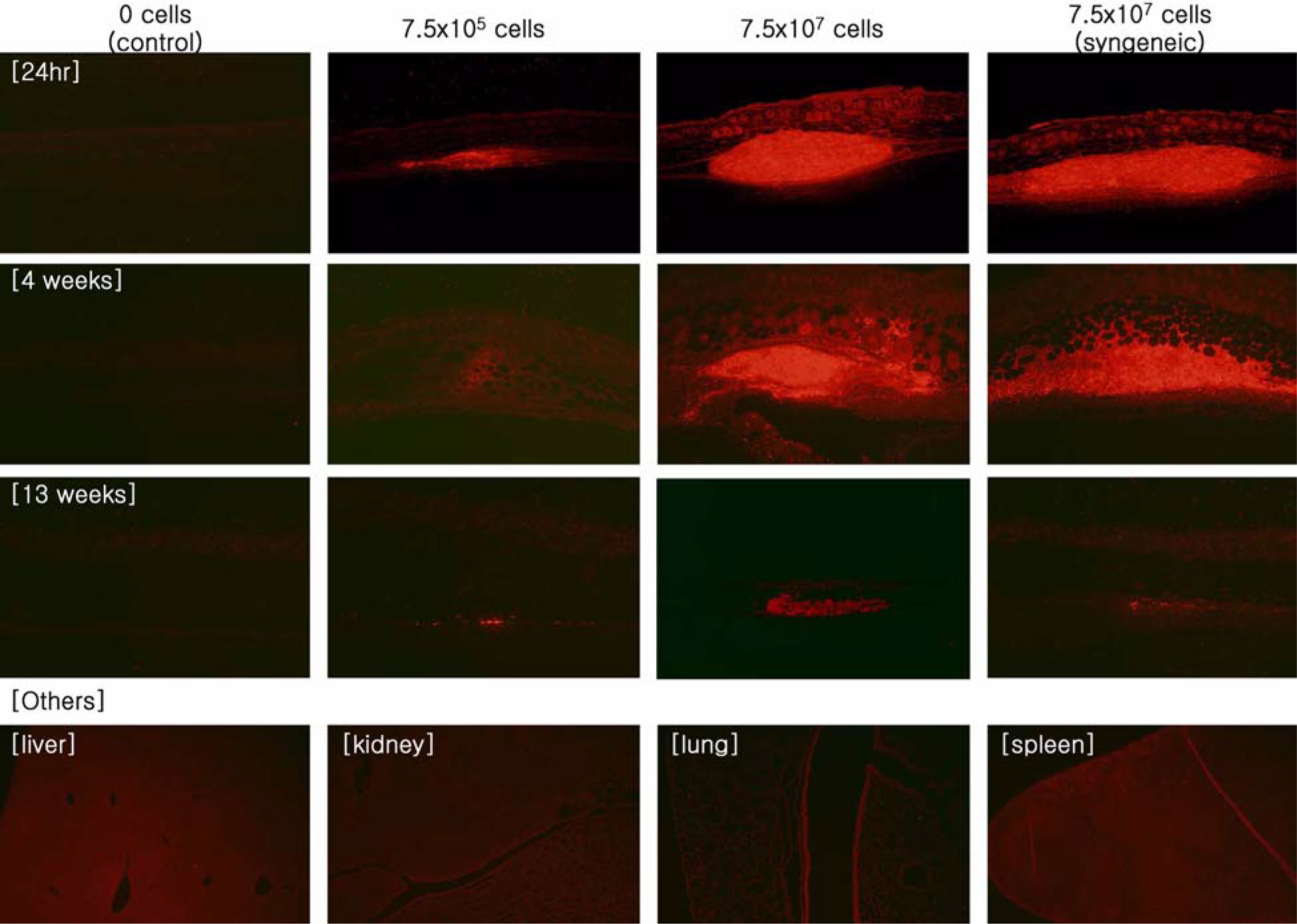
- Human fibroblasts were developed for cellular therapy with the aim of correcting of depressed scars, but the safety of that in vivo is unclear. In this study, we assessed the safety of human fibroblasts by investigating the tumorigenicity, 13-week toxicity and through distribution studies. In the tumorigenicity test, nude mice were divided into three dosage level treatment groups with a negative/positive control group. At 6 months after intradermal transplantation, all of the treatment groups showed no development of a nodule on the injection sites and organs. Toxicity studies were performed using ICR and BALB/c mice for 13 weeks. The mice were divided into three dosage level treatment groups with a control and a syngeneic group. There was no treatment-related effect on clinical signs, mortality, body weight, food/water consumption, hematology, serum biochemistry, urine, necropsy findings and histopathological findings in any groups. These results suggest that the no-observed-effect level (NOEL) of the human fibroblasts was greater than 7.5x10(7) cells/kg for mice. In the distribution study, groups were treated with fibroblasts labeled with a fluorescent dye (CM-DiI) at low and high doses with a control and a syngeneic group. At 24 hours, a large percentage of the labeled fibroblasts were observed at the dermal layer. At 3 months, fluorescence of the labeled fibroblasts continued to be observed. Other tissues were not detected the fluorescence at any time. These studies demonstrate that the safety of human fibroblasts is reasonable with no toxic effect, no tumorigenicity and retention in the dermis. Our studies define preclinical safety testing standards relevant to the development of cellular therapeutics.
Keyword
MeSH Terms
Figure
Reference
-
Adonai N.., Nguyen K.N.., Walsh J.., Iyer M.., Toyokuni T.., Phelps M.E.., McCarthy T.., McCarthy D.W.., Gambhir S.S.2002. Ex vivo cell labeling with 64Cu-pyruvaldehyde-bis(N4-methylthiosemicarbazone) for imaging cell trafficking in mice with positron-emission tomography. Proc. Natl. Acad. Sci. U.S.A. 99(5):3030–3035.
ArticleAllers C.., Sierralta W.D.., Neubauer S.., Rivera F.., Minguell J.J.., Conget P.A.2004. Dynamic of distribution of human bone marrow-derived mesenchymal stem cells after transplantation into adult unconditioned mice. Transplantation. 78(4):503–508.
ArticleBenten D.., Cheng K.., Gupta S.2006. Identification of transplanted human cells in animal tissues. Methods Mol. 326:189–201.
ArticleBoss W.K. Jr.., Usal H.., Chernoff G.., Keller G.S.., Lask G.P.., Fodor P.B.2000. Autologous cultured fibroblasts as cellular therapy in plastic surgery. Clin. Plast. Surg. 27(4):613–626.
ArticleBulte J.W.., Duncan I.D.., Frank J.A.2002. In vivo magnetic resonance tracking of magnetically labeled cells after transplantation. J. Cereb. Blood Flow Metab. 22(8):899–907.
ArticleCho J.J.., Malhi H.., Wang R.., Joseph B.., Ludlow J.W.., Susick R.., Gupta S.2002. Enzymatically labeled chromosomal probes for in situ identification of human cells in xenogeneic transplant models. Nat. Med. 8(9):1033–1036.
ArticleFerrari A.., Hannouche D.., Oudina K.., Bourguignon M.., Meunier A.., Sedel L.., Petite H.2001. In vivo tracking of bone marrow fibroblasts with fluorescent carbocyanine dye. J. Biomed. Mater. Res. 56(3):361–367.
ArticleFischer U.M.., Harting M.T.., Jimenez F.., Monzon-Posadas W.O.., Xue H.., Savitz S.I.., Laine G.A.., Cox, C.S. Jr.2009. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 18(5):683–692.
ArticleGalbraith D.N.2004. Regulatory and microbiological safety issues surrounding cell and tissue-engineering products. Biotechnol. Appl. Biochem. 40(Pt 1), 35-39. Guerret, S., Govignon, E., Hartmann, D.J. and Ronfard, V. (2003) Longterm remodeling of a bilayered living human skin equivalent (Apligraf) grafted onto nude mice: immuno-localization of human cells and characterization of extracellular matrix. Wound Repair Regen. 11(1):35–45.
ArticleHebda P.A.., Dohar J.E.1999. Transplanted fetal fibroblasts: survival and distribution over time in normal adult dermis compared with autogenic, allogenic, and xenogenic adult fibroblasts. Otolaryngol Head Neck Surg. 121(3):245–251.
ArticleKovacsovics-Bankowski M.., Mauch K.., Raber A.., Streeter P.R.., Deans R.J.., Maziarz R.T.., Van't Hof W.2008. Preclinical safety testing supporting clinical use of allogeneic multipotent adult progenitor cells. Cytotherapy. 10(7):730–742.
ArticleLawrenz B.., Schiller H.., Willbold E.., Ruediger M.., Muhs A.., Esser S.2004. Highly sensitive biosafety model for stem-cell-derived grafts. Cytotherapy. 6(3):212–222.
ArticleLee E.S.., Kwon E.A.., Park J.R.., Kang B.C.., Kang K.S.., Cho M.H.2007. Tumorigenesis study of canine adipose derived-mesenchymal stem cell. J. Toxicol. Pub. Health. 23(3):271–278.
ArticleLee J.H.., Kim Y.K.., Lee S.J.2006. Treatment of Aplasia Cuti Congenita using allogenic dermal matrix and cultured epithelial autograft: A Case Report. J. Korean Soc. Plast. Reconstr. Surg. 33(5):672–675.Liu L.., Sun Z.., Chen B.., Han Q.., Liao L.., Jia M.., Cao Y.., Ma J.., Sun Q.., Guo M.., Liu Z.., Ai H.., Zhao R.C.2006. Ex vivo expansion and in vivo infusion of bone marrow-derived Flk-1+CD31-CD34- mesenchymal stem cells: feasibility and safety from monkey to human. Stem Cells Dev. 15(3):349–357.
ArticleMeyerrose T.E.., De Ugarte D.A.., Hofling A.A.., Herrbrich P.E.., Cordonnier T.D.., Shultz L.D.., Eagon J.C.., Wirthlin L.., Sands M.S.., Hedrick M.A.., Nolta J.A.2007. In vivo distribution of human adipose-derived mesenchymal stem cells in novel xenotransplantation models. Stem Cells. 25(1):220–227.
ArticleMoeller F.., Nielsen F.C.., Nielsen L.B.2003. New tools for quantifying and visualizing adoptively transferred cells in recipient mice. J. Immunol. Methods 282(1-2), 73-82. Rubio, D., Garcia-Castro, J., Martin, M.C., de la Fuente, R., Cigudosa, J.C., Lloyd, A.C. and Bernad, A. (2005) Spontaneous human adult stem cell transformation. Cancer Res. 65(8):3035–3039.
ArticleSandulache V.C.., Zhou Z.., Sherman A.., Dohar J.E.., Hebda P.A.2003. Impact of transplanted fibroblasts on rabbit skin wounds. Arch. Otolaryngol. Head Neck Surg. 129(3):345–350.
ArticleSchrepfer S.., Deuse T.., Reichenspurner H.., Fischbein M.P.., Robbins R.C.., Pelletier M.P.2007. Stem cell transplantation: the lung barrier. Transplant. Proc. 39(2):573–576.
ArticleSeo S.W.., Chang C.H.., Cho M.S.., Hong Y.G.., Jeon S.W.2007. Treatment of partial thickness skin defect with cultured allogenic keratinocytes (Kaloderm.). J. Korean Soc. Traumatol. 20(1):1–5.Thompson M.., Wall D.M.., Hicks R.J.., Prince H.M.2005. In vivo tracking for cell therapies. Q. J. Nucl. Med. Mol. Imaging. 49(4):339–348.Trosko J.E.., Tai M.H.2006. Adult stem cell theory of the multi-stage, multi-mechanism theory of carcinogenesis: role of inflammation on the promotion of initiated stem cells. Contrib. Microbiol. 13:45–65.
ArticleVilalta M.., Degano I.R.., Bago J.., Gould D.., Santos M.., Garcia-Arranz M.., Ayats R.., Fuster C.., Chernajovsky Y.., Garcia-Olmo D.., Rubio N.., Blanco J.2008. Biodistribution, longterm survival, and safety of human adipose tissue-derived mesenchymal stem cells transplanted in nude mice by high sensitivity non-invasive bioluminescence imaging. Stem Cells Dev. 17(5):993–1003.
ArticleWatson D.., Keller G.S.., Lacombe V.., Fodor P.B.., Rawnsley J.., Lask G.P.1999. Autologous fibroblasts for treatment of facial rhytids and dermal depressions. A pilot study. Arch. Facial Plast. Surg. 1(3):165–170.
ArticleYoon S.H.., Shim J.S.., Jung J.M.., Park D.H.., Song C.H.2008. The usefulnesss of cultured allogenic keratinocyte for burn treatment. J. Korean Soc. Plast. Reconstr. Surg. 35(4):413–418.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of inhaled 1,2-dichlorobenzene on cytochrome P450s and lipid peroxidation in B6C3F1 mice
- Biological Safety Test of Seoultype Keratoprosthesis
- Guideline on safety evaluation of cell-based medicinal products for animal use
- Acute and 13-week subchronic toxicological evaluations of turanose in mice
- Histologic study of bone-forming capacity on polydeoxyribonucleotide combined with demineralized dentin matrix