J Dent Anesth Pain Med.
2015 Jun;15(2):47-52.
Considerations for submucosal midazolam administration in combination with oral and inhaled medications for sedation of pediatric dental patients
- Affiliations
-
- 1Department of Dentistry, Ajou University School of Medicine, Korea. pedobaek@nate.com
Abstract
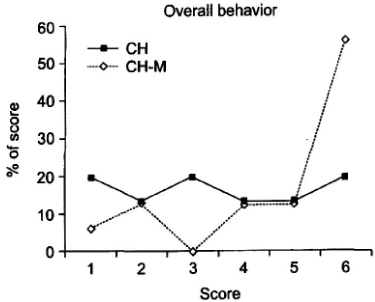
- Sedation allows patients to maintain their airway independently and respond appropriately to physical stimulation and verbal command while maintaining a minimum depressed level of consciousness. Drugs commonly used for sedation of pediatric dental patients include a combination of chloral hydrate, hydroxyzine, and nitrous oxide-oxygen. Midazolam is a benzodiazepine and currently one of the most commonly used intravenous sedative agents. It can be easily titrated to provide a wide range of sedation, from conscious sedation to deep sedation, and exhibits a wide safety margin without severe respiratory and circulatory depression. At an appropriate dose, it also decreases patient anxiety and induces amnesia. We found that the submucosal administration of midazolam combined with chloral hydrate provided increased sedative effects and decreased the postoperative vomiting response compared with conventional chloral hydrate administration, with no significant difference in physiological responses. The depth of sedation can be titrated using this technique.
MeSH Terms
-
Amnesia
Anxiety
Benzodiazepines
Chloral Hydrate
Conscious Sedation
Consciousness Disorders
Deep Sedation
Depression
Humans
Hydroxyzine
Hypnotics and Sedatives
Midazolam*
Pediatric Dentistry
Physical Stimulation
Postoperative Nausea and Vomiting
Benzodiazepines
Chloral Hydrate
Hydroxyzine
Hypnotics and Sedatives
Midazolam
Figure
Reference
-
1. An SY, Choi BJ, Kwak JY, Kang JW, Lee JH. A survey of sedation practices in the korean pediatric dental office. J Korean Acad Pediatr Dent. 2005; 32:444–453.2. Wilson S, McTigue DJ. Survey of conscious sedation practices in pediatric dentistry advanced residency programs. J Dent Educ. 1989; 53:595–597.
Article3. Carr KR, Wilson S, Nimer S, Thornton JB Jr. Behavior management techniques among pediatric dentists practicing in the southeastern United States. Pediatr Dent. 1999; 21:347–353.4. Dentistry AAOP. Guideline on behavior guidance for the pediatric dental patient. Pediatr Dent. 2011; 36:179–191.5. Dentistry AAOP. Guideline on the elective use of minimal, moderate, and deep sedation and general anesthesia for pediatric dental patients. Pediatr Dent. 2005; 27:110–118.6. Wilson S, Farrell K, Griffen A, Coury D. Conscious sedation experiences in graduate pediatric dentistry programs. Pediatr Dent. 2001; 23:307–314.7. Nathan JE. Management of the refractory young child with chloral hydrate: dosage selection. ASDC J Dent Child. 1987; 54:22–29.8. Avalos-Arenas V, Moyao-García D, Nava-Ocampo AA, Zayas-Carranza RE, Fragoso-Ríos R. Is chloral hydrate/ hydroxyzine a good option for paediatric dental outpatient sedation? Curr Med Res Opin. 1998; 14:219–226.
Article9. Wilson S, Matusak A, Casamassimo PS, Larsen P. The effects of nitrous oxide on pediatric dental patients sedated with chloral hydrate and hydroxyzine. Pediatr Dent. 1998; 20:253–258.10. Chowdhury J, Vargas KG. Comparison of chloral hydrate, meperidine, and hydroxyzine to midazolam regimens for oral sedation of pediatric dental patients. Pediatr Dent. 2005; 27:191–197.11. Myers GR, Maestrello CL, Mourino AP, Best AM. Effect of submucosal midazolam on behavior and physiologic response when combined with oral chloral hydrate and nitrous oxide sedation. Pediatr Dent. 2004; 26:37–43.12. Webb MD, Moore PA. Sedation for pediatric dental patients. Dent Clin North Am. 2002; 46:803–814.
Article13. Malamed SF. Sedation: A guide to patient management. 5th ed. Elsevier Health Sciences;2009. p. 90–93.14. Shapira J, Kupietzky A, Kadari A, Fuks AB, Holan G. Comparison of oral midazolam with and without hydroxyzine in the sedation of pediatric dental patients. Pediatr Dent. 2004; 26:492–496.15. Jensen B. Benzodiazepine sedation in paediatric dentistry. Swed Dent J Suppl. 2005; 153:1–45.16. Lam C, Udin RD, Malamed SF, Good DL, Forrest JL. Midazolam premedication in children: a pilot study comparing intramuscular and intranasal administration. Anesth Prog. 2005; 52:56–61.
Article17. Kupietzky A, Houpt MI. Midazolam: a review of its use for conscious sedation of children. Pediatr Dent. 1993; 15:237–241.18. Alfonzo-Echeverri E, Troutman KC, George W. Absorption and elimination of midazolam by submucosal and intramuscular routes. Anesth Prog. 1990; 37:277–281.19. Lee YE, Park MK, Kim SY, Kim YH, Jung SH, Baek KW. Sedation evaluation using BIS index assessment with and without the added submucosal midazolam. J Korean Acad Pediatr Dent. 2007; 34:91–98.20. Reves JG, Fragen RJ, Vinik HR, Greenblatt DJ. Midazolam: pharmacology and uses. Anesthesiology. 1985; 62:310–324.21. Persson P, Nilsson A, Hartvig P, Tamsen A. Pharmacokinetics of midazolam in total i.v. anaesthesia. Br J Anaesth. 1987; 59:548–556.22. Payne K, Mattheyse FJ, Liebenberg D, Dawes T. The pharmacokinetics of midazolam in paediatric patients. Eur J Clin Pharmacol. 1989; 37:267–272.
Article23. Lee YE, Park ME, Kim YH, Jung SH, Baek KW. The Sedative Effects of Submucosal Midazolam in Children. J Dent Anesth Pain Med. 2005; 5:101–106.
Article24. Park MK, Kim YH, Jung SH, Baek KW. Safety and Efficacy of Submucosal Midazolam When Combined with Oral Chloral Hydrate, Hydroxyzine and Nitrous Oxide Sedation by using Houpt's Scale. J Dent Anesth Pain Med. 2006; 6:103–112.
Article25. Koo JE, Baek KW. Postsedation events in pediatric patients sedated for dental treatment. J Korean Acad Pediatr Dent. 2009; 36:209–216.26. Kim YH, Jung SH, Baek KW. Comparison of Behavioral Response between Intranasal and Submucosal Midazolam Adminstration. J Korean Acad Pediatr Dent. 2008; 35:427–436.27. Pearson RC, McCloy RF, Morris P, Bardhan KD. Midazolam and flumazenil in gastroenterology. Acta Anaesthesiol Scand Suppl. 1990; 92:21–24.28. Sievers TD, Yee JD, Foley ME, Blanding PJ, Berde CB. Midazolam for conscious sedation during pediatric oncology procedures: safety and recovery parameters. Pediatrics. 1991; 88:1172–1179.
Article29. Griffin JW. Submucosal Sedation 5-year pilot study [abstract]. Anesth Prog. 2000; 47:88.30. Coulthard P, Sano K, Thomson PJ, Macfarlane TV. The effects of midazolam and flumazenil on psychomotor function and alertness in human volunteers. Br Dent J. 2000; 188:325–328.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Comparison of Behavioral Response of Additional Submucosal Midazolam with Oral Chloral Hydrate, Hydroxyzine and Nitrous Oxide for Pediatric Conscious Sedation
- Managing the behavior of a patient with autism by sedation via submucosal route during dental treatment
- Prospective clinical study of midazolam sedation in patients undergoing dental practice
- The Sedative Effects of Submucosal Midazolam in Children
- Effect of Submucosal Midazolam on Percutaneous Saturation Percentage of Oxygen (SpOâ‚‚), End-tidal Carbon Dioxide (EtCOâ‚‚) and Physiologic Response When Combined with Chloral Hydrate, Hydroxyzine and Nitrous Oxide Sedation