Endocrinol Metab.
2016 Jun;31(2):336-342. 10.3803/EnM.2016.31.2.336.
The Role of Nuclear Factor-E2-Related Factor 1 in the Oxidative Stress Response in MC3T3-E1 Osteoblastic Cells
- Affiliations
-
- 1Department of Internal Medicine, Cheil General Hospital & Women's Healthcare Center, Dankook University College of Medicine, Seoul, Korea.
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. lsk@yumc.yonsei.ac.kr
- KMID: 2308865
- DOI: http://doi.org/10.3803/EnM.2016.31.2.336
Abstract
- BACKGROUND
Reactive oxygen species (ROS) and antioxidants are associated with maintenance of cellular function and metabolism. Nuclear factor-E2-related factor 1 (NFE2L1, Nrf1) is known to regulate the expression of a number of genes involved in oxidative stress and inflammation. The purpose of this study was to examine the effects of NFE2L1 on the response to oxidative stress in osteoblastic MC3T3-E1 cells.
METHODS
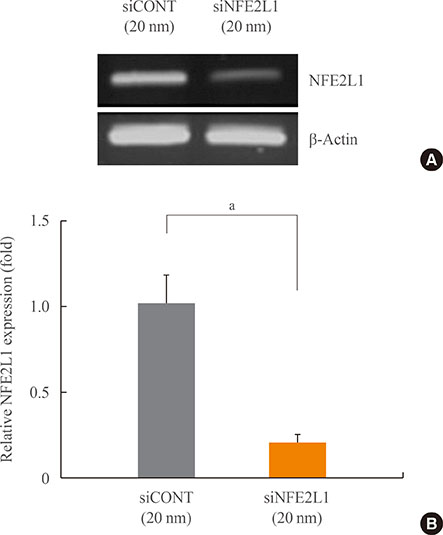
The murine calvaria-derived MC3T3-E1 cell line was exposed to lipopolysaccharide (LPS) for oxidative stress induction. NFE2L1 effects were evaluated using small interfering RNA (siRNA) for NFE2L1 mRNA. ROS generation and the levels of known antioxidant enzyme genes were assayed.
RESULTS
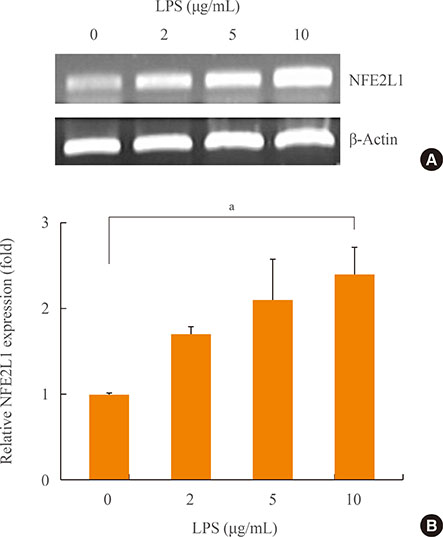
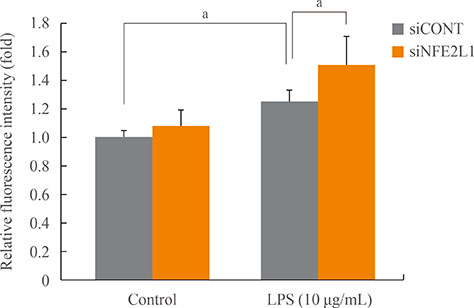
NFE2L1 expression was significantly increased 2.4-fold compared to the control group at 10 µg/mL LPS in MC3T3-E1 cells (P<0.05). LPS increased formation of intracellular ROS in MC3T3-E1 cells. NFE2L1 knockdown led to an additional increase of ROS (20%) in the group transfected with NFE2L1 siRNA compared with the control group under LPS stimulation (P<0.05). RNA interference of NFE2L1 suppressed the expression of antioxidant genes including metallothionein 2, glutamatecysteine ligase catalytic subunit, and glutathione peroxidase 1 in LPS-treated MC3T3-E1 cells.
CONCLUSION
Our results suggest that NFE2L1 may have a distinct role in the regulation of antioxidant enzymes under inflammation-induced oxidative stress in MC3T3-E1 osteoblastic cells.
MeSH Terms
-
Antioxidants
Catalytic Domain
Cell Line
Glutathione Peroxidase
Inflammation
Metabolism
Metallothionein
NF-E2-Related Factor 1
Osteoblasts*
Oxidative Stress*
Reactive Oxygen Species
RNA Interference
RNA, Messenger
RNA, Small Interfering
Antioxidants
Glutathione Peroxidase
Metallothionein
NF-E2-Related Factor 1
RNA, Messenger
RNA, Small Interfering
Reactive Oxygen Species
Figure
Reference
-
1. Banfi G, Iorio EL, Corsi MM. Oxidative stress, free radicals and bone remodeling. Clin Chem Lab Med. 2008; 46:1550–1555.2. Lyakhovich VV, Vavilin VA, Zenkov NK, Menshchikova EB. Active defense under oxidative stress. The antioxidant responsive element. Biochemistry (Mosc). 2006; 71:962–974.3. Sheweita SA, Khoshhal KI. Calcium metabolism and oxidative stress in bone fractures: role of antioxidants. Curr Drug Metab. 2007; 8:519–525.4. Maggio D, Barabani M, Pierandrei M, Polidori MC, Catani M, Mecocci P, et al. Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab. 2003; 88:1523–1527.5. Yalin S, Bagis S, Polat G, Dogruer N, Cenk Aksit S, Hatungil R, et al. Is there a role of free oxygen radicals in primary male osteoporosis? Clin Exp Rheumatol. 2005; 23:689–692.6. Schletter J, Heine H, Ulmer AJ, Rietschel ET. Molecular mechanisms of endotoxin activity. Arch Microbiol. 1995; 164:383–389.7. Nair SP, Meghji S, Wilson M, Reddi K, White P, Henderson B. Bacterially induced bone destruction: mechanisms and misconceptions. Infect Immun. 1996; 64:2371–2380.8. Kato N, Nakayama Y, Nakajima Y, Samoto H, Saito R, Yamanouchi F, et al. Regulation of bone sialoprotein (BSP) gene transcription by lipopolysaccharide. J Cell Biochem. 2006; 97:368–379.9. Chan JY, Han XL, Kan YW. Cloning of Nrf1, an NF-E2-related transcription factor, by genetic selection in yeast. Proc Natl Acad Sci U S A. 1993; 90:11371–11375.10. Biswas M, Chan JY. Role of Nrf1 in antioxidant response element-mediated gene expression and beyond. Toxicol Appl Pharmacol. 2010; 244:16–20.11. Lu SC, Ge JL, Kuhlenkamp J, Kaplowitz N. Insulin and glucocorticoid dependence of hepatic gamma-glutamylcysteine synthetase and glutathione synthesis in the rat. Studies in cultured hepatocytes and in vivo. J Clin Invest. 1992; 90:524–532.12. Kwong M, Kan YW, Chan JY. The CNC basic leucine zipper factor, Nrf1, is essential for cell survival in response to oxidative stress-inducing agents. Role for Nrf1 in gammagcs(l) and gss expression in mouse fibroblasts. J Biol Chem. 1999; 274:37491–37498.13. Lee TD, Yang H, Whang J, Lu SC. Cloning and characterization of the human glutathione synthetase 5'-flanking region. Biochem J. 2005; 390(Pt 2):521–528.14. Myhrstad MC, Husberg C, Murphy P, Nordstrom O, Blomhoff R, Moskaug JO, et al. TCF11/Nrf1 overexpression increases the intracellular glutathione level and can transactivate the gamma-glutamylcysteine synthetase (GCS) heavy subunit promoter. Biochim Biophys Acta. 2001; 1517:212–219.15. Ohtsuji M, Katsuoka F, Kobayashi A, Aburatani H, Hayes JD, Yamamoto M. Nrf1 and Nrf2 play distinct roles in activation of antioxidant response element-dependent genes. J Biol Chem. 2008; 283:33554–33562.16. Wang Y, Lou MF. The regulation of NADPH oxidase and its association with cell proliferation in human lens epithelial cells. Invest Ophthalmol Vis Sci. 2009; 50:2291–2300.17. Marriott I. Osteoblast responses to bacterial pathogens: a previously unappreciated role for bone-forming cells in host defense and disease progression. Immunol Res. 2004; 30:291–308.18. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007; 39:44–84.19. Chen L, Kwong M, Lu R, Ginzinger D, Lee C, Leung L, et al. Nrf1 is critical for redox balance and survival of liver cells during development. Mol Cell Biol. 2003; 23:4673–4686.20. Durnam DM, Hoffman JS, Quaife CJ, Benditt EP, Chen HY, Brinster RL, et al. Induction of mouse metallothionein-I mRNA by bacterial endotoxin is independent of metals and glucocorticoid hormones. Proc Natl Acad Sci U S A. 1984; 81:1053–1056.21. Durnam DM, Palmiter RD. Transcriptional regulation of the mouse metallothionein-I gene by heavy metals. J Biol Chem. 1981; 256:5712–5716.22. Mayo KE, Palmiter RD. Glucocorticoid regulation of metallothionein-I mRNA synthesis in cultured mouse cells. J Biol Chem. 1981; 256:2621–2624.23. Searle PF, Davison BL, Stuart GW, Wilkie TM, Norstedt G, Palmiter RD. Regulation, linkage, and sequence of mouse metallothionein I and II genes. Mol Cell Biol. 1984; 4:1221–1230.24. Beach LR, Palmiter RD. Amplification of the metallothionein-I gene in cadmium-resistant mouse cells. Proc Natl Acad Sci U S A. 1981; 78:2110–2114.25. Li DY, Xue MY, Geng ZR, Chen PY. The suppressive effects of Bursopentine (BP5) on oxidative stress and NF-κB activation in lipopolysaccharide-activated murine peritoneal macrophages. Cell Physiol Biochem. 2012; 29:9–20.26. Hernandez-Montes E, Pollard SE, Vauzour D, Jofre-Montseny L, Rota C, Rimbach G, et al. Activation of glutathione peroxidase via Nrf1 mediates genistein's protection against oxidative endothelial cell injury. Biochem Biophys Res Commun. 2006; 346:851–859.27. Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, et al. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem. 2000; 275:16023–16029.28. Moinova HR, Mulcahy RT. Up-regulation of the human gamma-glutamylcysteine synthetase regulatory subunit gene involves binding of Nrf-2 to an electrophile responsive element. Biochem Biophys Res Commun. 1999; 261:661–668.29. Kousteni S. FoxOs: unifying links between oxidative stress and skeletal homeostasis. Curr Osteoporos Rep. 2011; 9:60–66.30. Kim J, Xing W, Wergedal J, Chan JY, Mohan S. Targeted disruption of nuclear factor erythroid-derived 2-like 1 in osteoblasts reduces bone size and bone formation in mice. Physiol Genomics. 2010; 40:100–110.31. Xing W, Singgih A, Kapoor A, Alarcon CM, Baylink DJ, Mohan S. Nuclear factor-E2-related factor-1 mediates ascorbic acid induction of osterix expression via interaction with antioxidant-responsive element in bone cells. J Biol Chem. 2007; 282:22052–22061.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Spironolactone Attenuates Methylglyoxal-induced Cellular Dysfunction in MC3T3-E1 Osteoblastic Cells
- Anti-Oral Microbial Activity and Anti-Inflammatory Effects of Rosmarinic Acid in Lipopolysaccharide-Stimulated MC3T3-E1 Osteoblastic Cells on a Titanium Surface
- Glycyrrhiza uralensis (licorice) extracts increase cell proliferation and bone marker enzyme alkaline phosphatase activity in osteoblastic MC3T3-E1 cells
- Protective Effects of Ursolic Acid on Osteoblastic Differentiation via Activation of IER3/Nrf2
- Insulin growth factor binding protein-3 enhances dental implant osseointegration against methylglyoxal-induced bone deterioration in a rat model