Endocrinol Metab.
2016 Jun;31(2):328-335. 10.3803/EnM.2016.31.2.328.
The Effects of High Fat Diet and Resveratrol on Mitochondrial Activity of Brown Adipocytes
- Affiliations
-
- 1Division of Endocrinology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. ejlee423@yuhs.ac
- 2Department of Medical Oncology, The First Affiliated Hospital, Xinxiang Medical University, Weihui, China.
- KMID: 2308864
- DOI: http://doi.org/10.3803/EnM.2016.31.2.328
Abstract
- BACKGROUND
Resveratrol (RSV) is a polyphenolic phytoalexin that has many effects on metabolic diseases such as diabetes and obesity. Given the importance of brown adipose tissue (BAT) for energy expenditure, we investigated the effects of RSV on brown adipocytes.
METHODS
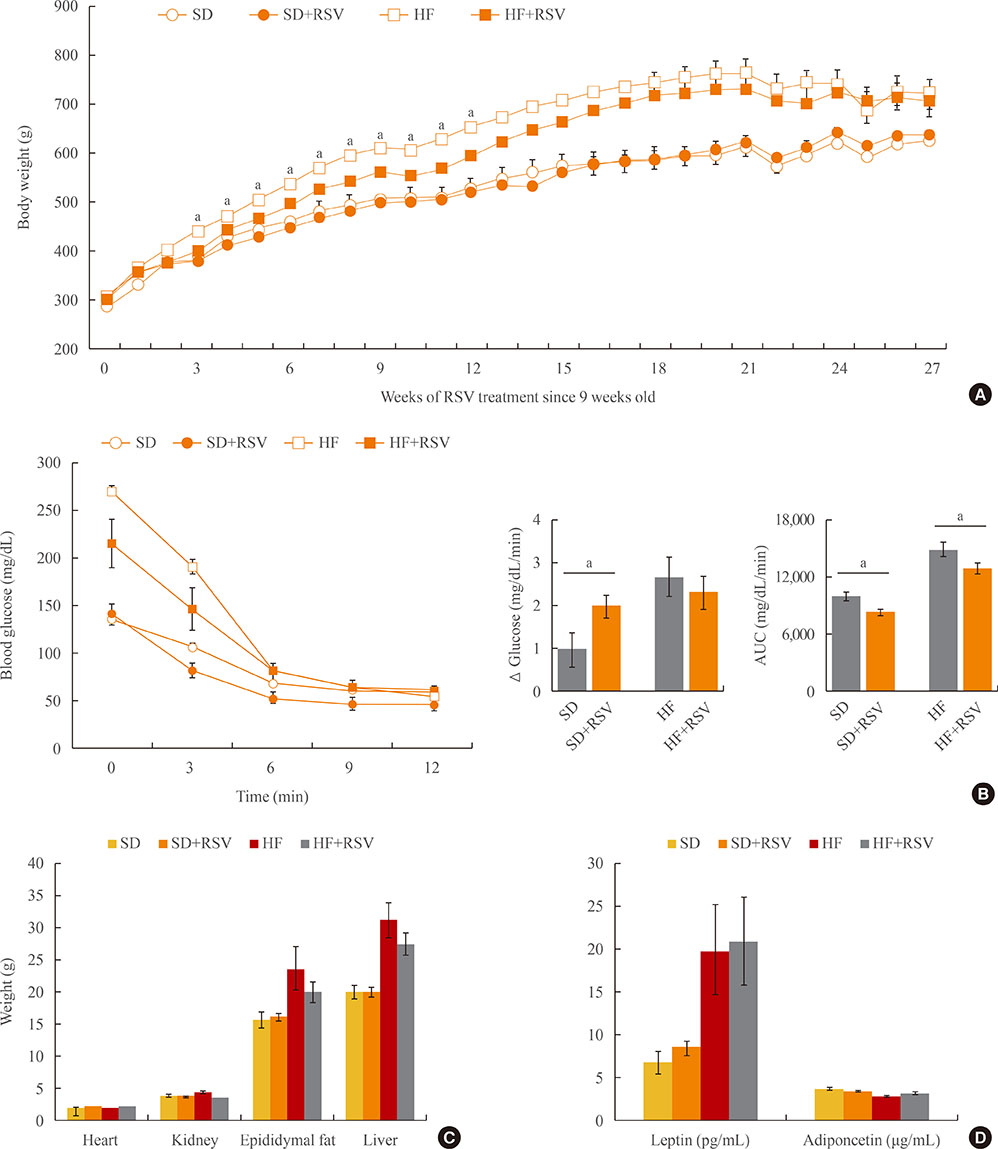
For the in vitro study, interscapular BAT was isolated from 7-week-old male Sprague Dawley rats. For the in vivo study, 7-week-old male Otsuka Long Evans Tokushima Fatty (OLETF) rats were divided into four groups and treated for 27 weeks with: standard diet (SD); SD+RSV (10 mg/kg body weight, daily); high fat diet (HFD); HFD+RSV. RSV was provided via oral gavage once daily during the in vivo experiments.
RESULTS
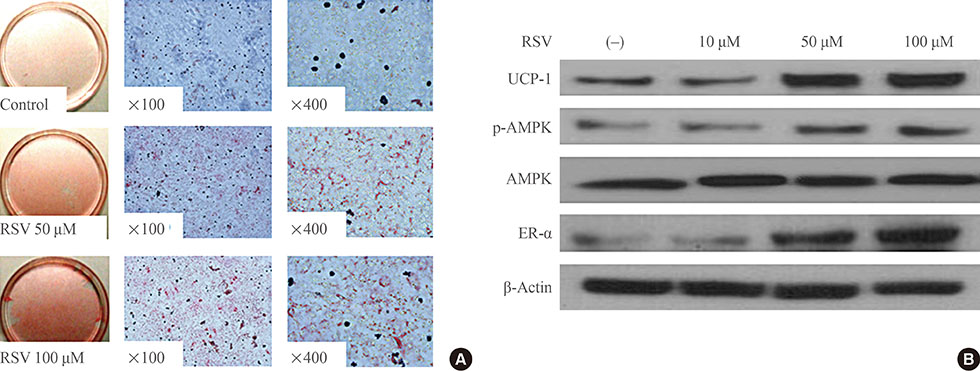
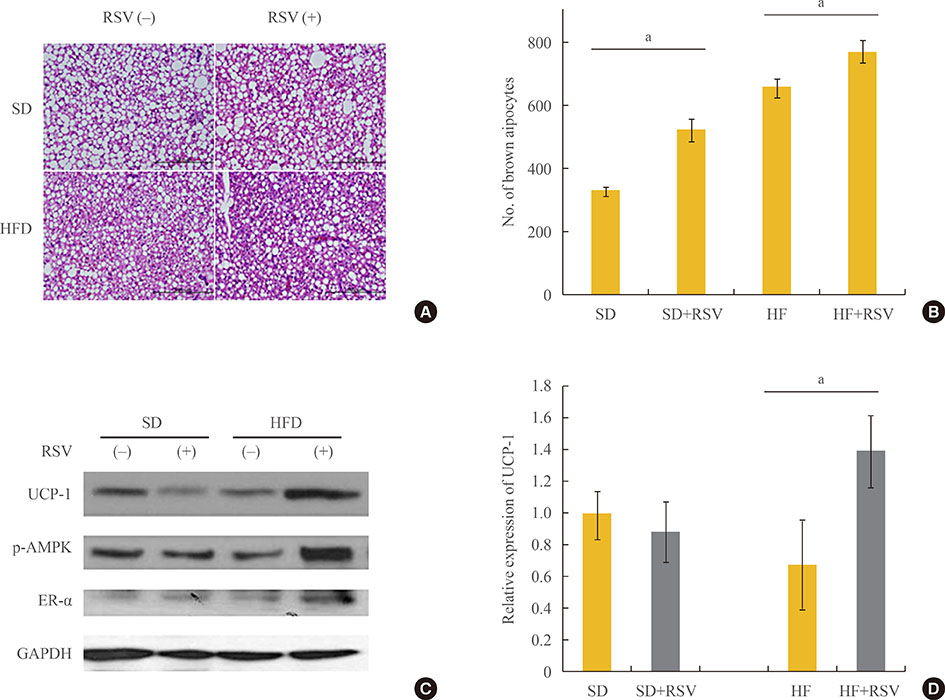
RSV treatment of primary cultured brown preadipocytes promoted mitochondrial activity, along with over-expression of estrogen receptor α (ER-α). In OLETF rats, both HFD and RSV treatment increased the weight of BAT and the differentiation of BAT. However, only RSV increased the mitochondrial activity and ER-α expression of BAT in the HFD-fed group. Finally, RSV improved the insulin sensitivity of OLETF rats by increasing the mitochondrial activity of BAT, despite having no effects on white adipocytes and muscles in either diet group.
CONCLUSION
RSV could improve insulin resistance, which might be associated with mitochondrial activity of brown adipocyte. Further studies evaluating the activity of RSV for both the differentiation and mitochondrial activity of BAT could be helpful in investigating the effects of RSV on metabolic parameters.
MeSH Terms
-
Adipocytes, Brown*
Adipocytes, White
Adipose Tissue, Brown
Animals
Body Weight
Diet
Diet, High-Fat*
Energy Metabolism
Estrogen Receptor alpha
Estrogens
Humans
In Vitro Techniques
Insulin Resistance
Male
Metabolic Diseases
Mitochondria
Muscles
Obesity
Rats
Rats, Inbred OLETF
Rats, Sprague-Dawley
Estrogen Receptor alpha
Estrogens
Figure
Reference
-
1. Nicholls DG, Locke RM. Thermogenic mechanisms in brown fat. Physiol Rev. 1984; 64:1–64.2. Trayhurn P, Milner RE. A commentary on the interpretation of in vitro biochemical measures of brown adipose tissue thermogenesis. Can J Physiol Pharmacol. 1989; 67:811–819.3. Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, Cannon B. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta. 2001; 1504:82–106.4. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009; 360:1509–1517.5. Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009; 58:1526–1531.6. Cos P, De Bruyne T, Apers S, Vanden Berghe D, Pieters L, Vlietinck AJ. Phytoestrogens: recent developments. Planta Med. 2003; 69:589–599.7. Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006; 444:337–342.8. Olholm J, Paulsen SK, Cullberg KB, Richelsen B, Pedersen SB. Anti-inflammatory effect of resveratrol on adipokine expression and secretion in human adipose tissue explants. Int J Obes (Lond). 2010; 34:1546–1553.9. Miranda S, Gonzalez-Rodriguez A, Revuelta-Cervantes J, Rondinone CM, Valverde AM. Beneficial effects of PTP1B deficiency on brown adipocyte differentiation and protection against apoptosis induced by pro- and anti-inflammatory stimuli. Cell Signal. 2010; 22:645–659.10. Andrade JM, Frade AC, Guimaraes JB, Freitas KM, Lopes MT, Guimaraes AL, et al. Resveratrol increases brown adipose tissue thermogenesis markers by increasing SIRT1 and energy expenditure and decreasing fat accumulation in adipose tissue of mice fed a standard diet. Eur J Nutr. 2014; 53:1503–1510.11. Klein J, Fasshauer M, Ito M, Lowell BB, Benito M, Kahn CR. Beta(3)-adrenergic stimulation differentially inhibits insulin signaling and decreases insulin-induced glucose uptake in brown adipocytes. J Biol Chem. 1999; 274:34795–34802.12. Kim WK, Choi HR, Park SG, Ko Y, Bae KH, Lee SC. Myostatin inhibits brown adipocyte differentiation via regulation of Smad3-mediated beta-catenin stabilization. Int J Biochem Cell Biol. 2012; 44:327–334.13. Lee P, Swarbrick MM, Ho KK. Brown adipose tissue in adult humans: a metabolic renaissance. Endocr Rev. 2013; 34:413–438.14. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004; 84:277–359.15. Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006; 27:728–735.16. Vegiopoulos A, Muller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A, et al. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science. 2010; 328:1158–1161.17. Lowell BB, S-Susulic V, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993; 366:740–742.18. Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011; 14:324–338.19. Carmona MC, Hondares E, Rodriguez de la Concepcion ML, Rodriguez-Sureda V, Peinado-Onsurbe J, Poli V, et al. Defective thermoregulation, impaired lipid metabolism, but preserved adrenergic induction of gene expression in brown fat of mice lacking C/EBPbeta. Biochem J. 2005; 389(Pt 1):47–56.20. Konings E, Timmers S, Boekschoten MV, Goossens GH, Jocken JW, Afman LA, et al. The effects of 30 days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. Int J Obes (Lond). 2014; 38:470–473.21. Beaudoin MS, Snook LA, Arkell AM, Simpson JA, Holloway GP, Wright DC. Resveratrol supplementation improves white adipose tissue function in a depot-specific manner in Zucker diabetic fatty rats. Am J Physiol Regul Integr Comp Physiol. 2013; 305:R542–R551.22. Cho SJ, Jung UJ, Choi MS. Differential effects of low-dose resveratrol on adiposity and hepatic steatosis in diet-induced obese mice. Br J Nutr. 2012; 108:2166–2175.23. Li Y, Wong K, Giles A, Jiang J, Lee JW, Adams AC, et al. Hepatic SIRT1 attenuates hepatic steatosis and controls energy balance in mice by inducing fibroblast growth factor 21. Gastroenterology. 2014; 146:539–549.e7.24. Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Ppargamma. Cell. 2012; 150:620–632.25. Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006; 5:493–506.26. Chow HH, Garland LL, Heckman-Stoddard BM, Hsu CH, Butler VD, Cordova CA, et al. A pilot clinical study of resveratrol in postmenopausal women with high body mass index: effects on systemic sex steroid hormones. J Transl Med. 2014; 12:223.27. Choi DK, Oh TS, Choi JW, Mukherjee R, Wang X, Liu H, et al. Gender difference in proteome of brown adipose tissues between male and female rats exposed to a high fat diet. Cell Physiol Biochem. 2011; 28:933–948.28. Rodriguez-Cuenca S, Monjo M, Frontera M, Gianotti M, Proenza AM, Roca P. Sex steroid receptor expression profile in brown adipose tissue. Effects of hormonal status. Cell Physiol Biochem. 2007; 20:877–886.29. Franco JG, Lisboa PC, da Silva Lima N, Peixoto-Silva N, Maia LA, Oliveira E, et al. Resveratrol prevents hyperleptinemia and central leptin resistance in adult rats programmed by early weaning. Horm Metab Res. 2014; 46:728–735.30. Gómez-Zorita S, Fernandez-Quintela A, Lasa A, Hijona E, Bujanda L, Portillo MP. Effects of resveratrol on obesity-related inflammation markers in adipose tissue of genetically obese rats. Nutrition. 2013; 29:1374–1380.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Response: The Effects of High Fat Diet and Resveratrol on Mitochondrial Activity of Brown Adipocytes (Endocrinol Metab 2016;31:328-35, Cheol Ryong Ku et al.)
- Letter: The Effects of High Fat Diet and Resveratrol on Mitochondrial Activity of Brown Adipocytes (Endocrinol Metab 2016;31:328-35, Cheol Ryong Ku et al.)
- High-fat diet alters the thermogenic gene expression to β-agonists or 18-carbon fatty acids in adipocytes derived from the white and brown adipose tissue of mice
- Brown Fat and Browning for the Treatment of Obesity and Related Metabolic Disorders
- Danshen Extracts Prevents Obesity and Activates Mitochondrial Function in Brown Adipose Tissue