Korean J Urol.
2006 Jun;47(6):656-660. 10.4111/kju.2006.47.6.656.
The Anti-inflammatory Effect of Cobalt-Protoporphyrin for Rats with Epididymitis Induced by Escherichia coli Infection
- Affiliations
-
- 1Department of Urology, Wonkwang University School of Medicine, Iksan, Korea. seraph@wonkwang.ac.kr
- KMID: 2294243
- DOI: http://doi.org/10.4111/kju.2006.47.6.656
Abstract
-
PURPOSE: Heme oxygenase-1 (HO-1), an inducible heat shock protein, catalyzes the heme to iron, biliverdin and carbon monoxide. It also has an inhibitory effect on necrosis and inflammation. Cobalt (III)-protoporphyrin IX (CoPP) is known to be a HO-1 inducer. Our intension was to find whether CoPP has an anti-inflammatory effect through the induction of HO-1 in rats with epididymitis.
MATERIALS AND METHODS
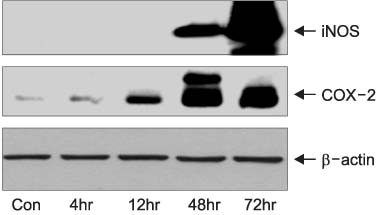
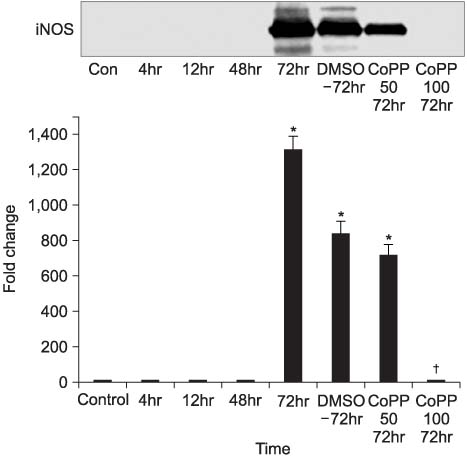
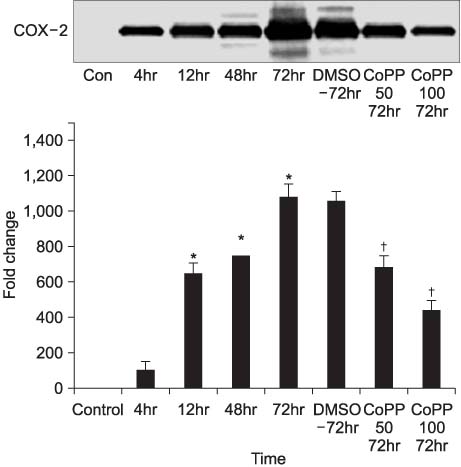
Thirty two Sprague-Dawley male rats (age: 8-12 weeks, weight: 200-250gm) were selected for the experiments. Anesthesia was performed with an intraperitoneal injection of ketamine hydrochloride (140mg/kg). Four rats were taken and used as a control group. Epididymitis was induced in 28 rats by an injection of E. coli (1 x 10(5)/ml) to the epididymis. In the first step, groups of 4 rats were sacrificed serially after 4, 12, 48, and 72 hours for Hematoxylin & Eosin (H&E) staining and Western blot for inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2. In the second step, groups of 4 rats were injected with either dimethyl sulphoxide (DMSO) 7 microliter, DMSO 7 microliter with 50mg/ml CoPP or DMSO 7 microliter with 100mg/ml CoPP. They were then sacrificed 72 hours later for H&E staining and Western blot for iNOS and COX-2.
RESULTS
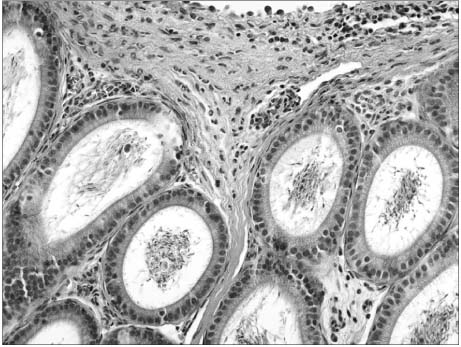
In the first step, increased inflammation was evident H&E staining over time. Western blots, iNOS expression was detected after 48 hours and COX-2 was after 12 hours. In the second step, decreased inflammation was evident H&E staining, and the expressions of iNOS and COX-2 were suppressed in the CoPP treated group.
CONCLUSIONS
CoPP can reduce the inflammation of epididymis in rats, and the mechanism may be related with HO-1.
Keyword
MeSH Terms
-
Anesthesia
Animals
Biliverdine
Blotting, Western
Carbon Monoxide
Cobalt
Dimethyl Sulfoxide
Eosine Yellowish-(YS)
Epididymis
Epididymitis*
Escherichia coli Infections*
Escherichia coli*
Escherichia*
Heat-Shock Proteins
Hematoxylin
Heme
Heme Oxygenase (Decyclizing)
Heme Oxygenase-1
Humans
Inflammation
Injections, Intraperitoneal
Iron
Ketamine
Male
Necrosis
Nitric Oxide Synthase Type II
Prostaglandin-Endoperoxide Synthases
Rats*
Rats, Sprague-Dawley
Biliverdine
Carbon Monoxide
Cobalt
Dimethyl Sulfoxide
Eosine Yellowish-(YS)
Heat-Shock Proteins
Hematoxylin
Heme
Heme Oxygenase (Decyclizing)
Heme Oxygenase-1
Iron
Ketamine
Nitric Oxide Synthase Type II
Prostaglandin-Endoperoxide Synthases
Figure
Reference
-
1. Torti PM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002. 99:3505–3516.2. Montellano PR. The mechanism of heme oxygenase. Curr Opin Chem Biol. 2000. 4:221–227.3. Applegate LA, Luscher P, Tyrrell RM. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res. 1991. 51:974–978.4. Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997. 37:517–554.5. Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002. 8:240–246.6. Brasile L, Buelow R, Stubenitsky BM, Kootstra G. Induction of heme oxygenase-1 in kidneys during ex vivo warm perfusion. Transplantation. 2003. 76:1145–1149.7. Wagener FA, Volk HD, Willis D, Abraham NG, Soares MP, Adema GJ, et al. Different faces of the heme-heme oxygenase system in inflammation. Pharm Rev. 2003. 55:551–571.8. Choi BM, Pae HO, Jeong YR, Oh GS, Jun CD, Kim BR, et al. Overexpression of heme oxygenase (HO-1) renders Jurkat T cells resistant to fas-mediated apoptosis: involvement of iron released by HO-1. Free Radical Biol Med. 2004. 36:858–871.9. Weber CM, Eke BC, Maines MD. Corticosterone regulates heme oxygenase-2 and NO synthase transcription and protein expression in rat brain. J Neurochem. 1994. 63:953–962.10. McCoubrey WK Jr, Huang TJ, Maines MD. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur J Biochem. 1997. 247:725–732.11. Siow RC, Sato H, Mann GE. Heme oxygenase carbon monoxide signalling pathway in atherosclerosis: anti-atherogenic actions of bilirubin and carbon monoxide? Cardiovasc Res. 1999. 41:385–394.12. Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad USA. 1968. 61:748–755.13. McCarter SD, Akyea TG, Lu X, Bihari A, Scott JR, Badhwar A, et al. Endogenous heme oxygenase induction is a critical mechanism attenuating apoptosis and restoring microvascular perfusion following limb ischemia/reperfusion. Surgery. 2004. 136:67–75.14. Gopinathan V, Miller NJ, Milner AD, Rice-Evans CA. Bilirubin and ascorbate antioxidant activity in neonatal plasma. FEBS Lett. 1994. 349:197–200.15. Choi BM, Pae HO, Kim YM, Chung HT. Nitric oxide-mediated cytoprotection of hepatocytes from glucose deprivation-induced cytotoxicity: involvement of heme oxygenase-1. Hepatology. 2003. 37:810–823.16. Wagener FA, Eggert A, Boerman OC, Oyen WJ, Verhofstad A, Abraham NG, et al. Heme is a potent inducer of inflammation in mice and is counteracted by heme oxygenase. Blood. 2001. 98:1802–1811.17. Sass G, Soares MC, Yamashita K, Seyfried S, Zimmermann WH, Eschenhagen T, et al. Heme oxygenase-1 and its reaction product, carbon monoxide, prevent inflammation-related apoptotic liver damage in mice. Hepatology. 2003. 38:909–918.18. Hayashi S, Takamiya R, Yamaguchi T, Matsumoto K, Tojo SJ, Tamatani T, et al. Induction of heme oxygenase-1 suppresses venular leukocyte adhesion elicited by oxidative stress: role of bilirubin generated by the enzyme. Circ Res. 1999. 85:663–671.19. Suttner DM, Dennery PA. Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J. 1999. 13:1800–1809.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Xanthogranulomatous Epididymitis Unresponsive to Antibiotic Treatment
- Escherichia coli O157: H7 Infection
- The Effect of Intravenous E. coli Injection on the Kidney after Ureteral Ligation in the Rat
- Escherichia coli O157:H7 Infection
- An outbreak of inapparent non-O157 enterohemorrhagic escherichia coli infection