Neuropathologic and Clinical Features of Human Medial Temporal Lobe Epilepsy
- Affiliations
-
- 1Stroke & Stem Cell Laboratory in Clinical Research Institute, Stem Cell Research Center, Department of Neurology, Seoul National University, Seoul, Korea. sangunlee@dreamwiz.com, rohjk@snu.ac.kr
- 2Program in Neuroscience, Neuroscience Research Institute of SNUMRC, Seoul National University, Seoul, Korea.
- 3Comprehensive Epilepsy Center, Seoul National University Hospital, Seoul, Korea.
- 4Department of Neurology, Seoul Paik Hospital, Inje University College of Medicine, Seoul, Korea.
- 5Department of Neurosurgery, Seoul National University Hospital, Seoul, Korea.
- KMID: 2287637
- DOI: http://doi.org/10.3988/jcn.2010.6.2.73
Abstract
- BACKGROUND AND PURPOSE
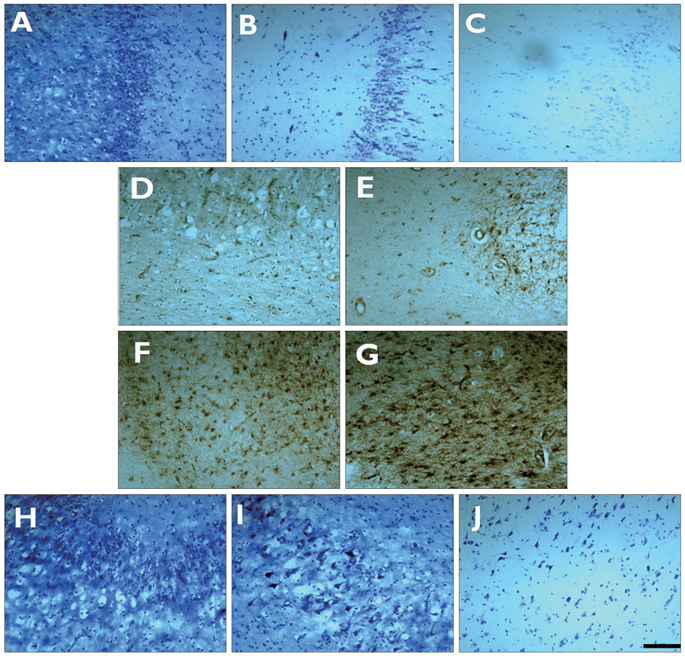
There is recent evidence of various types of morphological changes in the hippocampus of a rodent model of medial temporal lobe epilepsy (mTLE). However, little is known about such changes in humans. We examined the histological changes [i.e., neuronal loss, cell genesis, and granule cell dispersion (GCD)] in surgical hippocampal specimens taken from patients with mTLE.
METHODS
Nissl staining, and nestin and Prox1 immunohistochemistry were performed on human hippocampal specimens obtained from patients with medically intractable mTLE, thus allowing the analysis of neuronal loss, cell genesis, and GCD, respectively. We also assessed the correlations between clinical parameters and the histopathologic findings.
RESULTS
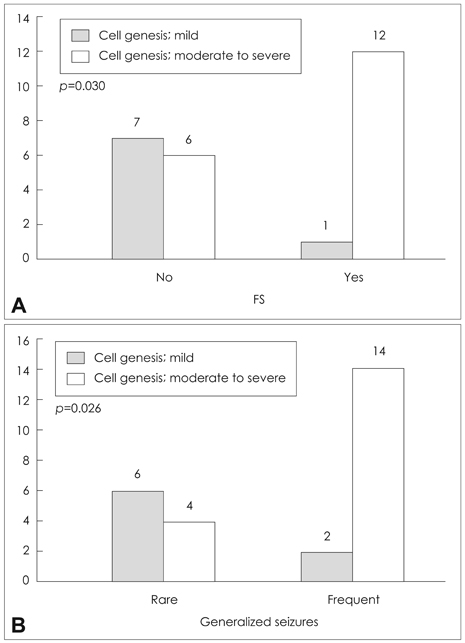
The degree of cell genesis in the granule cell layer was significantly correlated with the severity of GCD, history of childhood febrile seizures, and frequent generalized seizures. Cell genesis was not correlated with cell death, age at seizure onset, duration of epilepsy, or the mean frequency of all seizures.
CONCLUSIONS
Our results indicate that cell genesis in the dentate gyrus of patients with mTLE is associated with GCD and is influenced by the presence of febrile seizures during childhood and the frequency of episodes of generalized seizures.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Asymmetric Gray Matter Volume Changes Associated with Epilepsy Duration and Seizure Frequency in Temporal-Lobe-Epilepsy Patients with Favorable Surgical Outcome
Jeong Sik Kim, Dae Lim Koo, Eun Yeon Joo, Sung Tae Kim, Dae Won Seo, Seung Bong Hong
J Clin Neurol. 2016;12(3):323-331. doi: 10.3988/jcn.2016.12.3.323.Clinical Utility of Interictal High-Frequency Oscillations Recorded with Subdural Macroelectrodes in Partial Epilepsy
Jounhong Ryan Cho, Eun Yeon Joo, Dae Lim Koo, Seung Chyul Hong, Seung Bong Hong
J Clin Neurol. 2012;8(1):22-34. doi: 10.3988/jcn.2012.8.1.22.Clinical Utility of Interictal High-Frequency Oscillations Recorded with Subdural Macroelectrodes in Partial Epilepsy
Jounhong Ryan Cho, Eun Yeon Joo, Dae Lim Koo, Seung Chyul Hong, Seung Bong Hong
J Clin Neurol. 2012;8(1):22-34. doi: 10.3988/jcn.2012.8.1.22.
Reference
-
1. Babb TL, Brown WJ. Engel J, editor. Pathological findings in epilepsy. 1987. New York: Raven Press;511–540.2. Meencke HJ, Veith G. Luders H, editor. Hippocampal sclerosis in epilepsy. Epilepsy surgery. 1991. New York: Raven Press;705–715.3. Houser CR. Granule cell dispersion in the dentate gyrus of humans with temporal lobe epilepsy. Brain Res. 1990. 535:195–204.
Article4. Lurton D, El Bahh B, Sundstrom L, Rougier A. Granule cell dispersion is correlated with early epileptic events in human temporal lobe epilepsy. J Neurol Sci. 1998. 154:133–136.
Article5. El Bahh B, Lespinet V, Lurton D, Coussemacq M, Le Gal La Salle G, Rougier A. Correlations between granule cell dispersion, mossy fiber sprouting, and hippocampal cell loss in temporal lobe epilepsy. Epilepsia. 1999. 40:1393–1401.
Article6. Thom M, Sisodiya SM, Beckett A, Martinian L, Lin WR, Harkness W, et al. Cytoarchitectural abnormalities in hippocampal sclerosis. J Neuropathol Exp Neurol. 2002. 61:510–519.
Article7. Blümcke I, Thom M, Wiestler OD. Ammon's horn sclerosis: a maldevelopmental disorder associated with temporal lobe epilepsy. Brain Pathol. 2002. 12:199–211.
Article8. Cavanagh JB, Meyer A. Aetiological aspects of Ammon's horn sclerosis associated with temporal lobe epilepsy. Br Med J. 1956. 2:1403–1407.
Article9. Dam AM. Epilepsy and neuron loss in the hippocampus. Epilepsia. 1980. 21:617–629.
Article10. Babb TL, Brown WJ, Pretorius J, Davenport C, Lieb JP, Crandall PH. Temporal lobe volumetric cell densities in temporal lobe epilepsy. Epilepsia. 1984. 25:729–740.
Article11. Mathern GW, Adelson PD, Cahan LD, Leite JP. Hippocampal neuron damage in human epilepsy: Meyer's hypothesis revisited. Prog Brain Res. 2002. 135:237–251.
Article12. Fuerst D, Shah J, Kupsky WJ, Johnson R, Shah A, Hayman-Abello B, et al. Volumetric MRI, pathological, and neuropsychological progression in hippocampal sclerosis. Neurology. 2001. 57:184–188.
Article13. Thom M, Zhou J, Martinian L, Sisodiya S. Quantitative post-mortem study of the hippocampus in chronic epilepsy: seizures do not inevitably cause neuronal loss. Brain. 2005. 128:1344–1357.
Article14. Jessberger S, Römer B, Babu H, Kempermann G. Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells. Exp Neurol. 2005. 196:342–351.
Article15. Fahrner A, Kann G, Flubacher A, Heinrich C, Freiman TM, Zentner J, et al. Granule cell dispersion is not accompanied by enhanced neurogenesis in temporal lobe epilepsy patients. Exp Neurol. 2007. 203:320–332.
Article16. Thom M, Martinian L, Williams G, Stoeber K, Sisodiya SM. Cell proliferation and granule cell dispersion in human hippocampal sclerosis. J Neuropathol Exp Neurol. 2005. 64:194–201.
Article17. Wiebe S, Blume WT, Girvin JP, Eliasziw M. Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001. 345:311–318.
Article18. Mathern GW, Babb TL, Vickrey BG, Melendez M, Pretorius JK. The clinical-pathogenic mechanisms of hippocampal neuron loss and surgical outcomes in temporal lobe epilepsy. Brain. 1995. 118:105–118.
Article19. Chu K, Kim M, Jung KH, Jeon D, Lee ST, Kim J, et al. Human neural stem cell transplantation reduces spontaneous recurrent seizures following pilocarpine-induced status epilepticus in adult rats. Brain Res. 2004. 1023:213–221.
Article20. Jung KH, Chu K, Kim M, Jeong SW, Song YM, Lee ST, et al. Continuous cytosine-b-D-arabinofuranoside infusion reduces ectopic granule cells in adult rat hippocampus with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Eur J Neurosci. 2004. 19:3219–3226.
Article21. Jung KH, Chu K, Lee ST, Kim J, Sinn DI, Kim JM, et al. Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiol Dis. 2006. 23:237–246.
Article22. Kruglyakova EP, Khovryakov AV, Shikhanov NP, Maccann GM 2nd, Vael I, Kruglyakov PP, et al. Nestin-expressing cells in the human hippocampus. Neurosci Behav Physiol. 2005. 35:891–897.
Article23. Pleasure SJ, Collins AE, Lowenstein DH. Unique expression patterns of cell fate molecules delineate sequential stages of dentate gyrus development. J Neurosci. 2000. 20:6095–6105.
Article24. Elliott RC, Khademi S, Pleasure SJ, Parent JM, Lowenstein DH. Differential regulation of basic helix-loop-helix mRNAs in the dentate gyrus following status epilepticus. Neuroscience. 2001. 106:79–88.
Article25. Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, et al. The chemokine SDF1 regulates migration of dentate granule cells. Development. 2002. 129:4249–4260.
Article26. Parent JM, Elliott RC, Pleasure SJ, Barbaro NM, Lowenstein DH. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann Neurol. 2006. 59:81–91.
Article27. McCloskey DP, Hintz TM, Pierce JP, Scharfman HE. Stereological methods reveal the robust size and stability of ectopic hilar granule cells after pilocarpine-induced status epilepticus in the adult rat. Eur J Neurosci. 2006. 24:2203–2210.
Article28. Pirttilä TJ, Manninen A, Jutila L, Nissinen J, Kälviäinen R, Vapalahti M, et al. Cystatin C expression is associated with granule cell dispersion in epilepsy. Ann Neurol. 2005. 58:211–223.
Article29. Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996. 16:2027–2033.
Article30. Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998. 4:1313–1317.
Article31. Heinrich C, Nitta N, Flubacher A, Müller M, Fahrner A, Kirsch M, et al. Reelin deficiency and displacement of mature neurons, but not neurogenesis, underlie the formation of granule cell dispersion in the epileptic hippocampus. J Neurosci. 2006. 26:4701–4713.
Article32. Scharfman HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J Neurosci. 2000. 20:6144–6158.
Article33. Gray WP, Sundstrom LE. Kainic acid increases the proliferation of granule cell progenitors in the dentate gyrus of the adult rat. Brain Res. 1998. 790:52–59.
Article34. Parent JM, Janumpalli S, McNamara JO, Lowenstein DH. Increased dentate granule cell neurogenesis following amygdala kindling in the adult rat. Neurosci Lett. 1998. 247:9–12.
Article35. Blümcke I, Schewe JC, Normann S, Brüstle O, Schramm J, Elger CE, et al. Increase of nestin-immunoreactive neural precursor cells in the dentate gyrus of pediatric patients with early-onset temporal lobe epilepsy. Hippocampus. 2001. 11:311–321.
Article36. Takei H, Wilfong A, Yoshor D, Armstrong DL, Bhattacharjee MB. Evidence of increased cell proliferation in the hippocampus in children with Ammon's horn sclerosis. Pathol Int. 2007. 57:76–81.
Article37. Dubé C, Richichi C, Bender RA, Chung G, Litt B, Baram TZ. Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis. Brain. 2006. 129:911–922.
Article38. Cendes F, Andermann F. Baram TZ, Shinnar S, editors. Do febrile seizures: promote temporal lobe epilepsy? Retrospective studies. 2002. San Diego: Academic Press;78–88.39. Dube CM, Brewster AL, Richichi C, Zha Q, Baram TZ. Fever, febrile seizures and epilepsy. Trends Neurosci. 2007. 30:490–496.
Article40. Lemmens EM, Lubbers T, Schijns OE, Beuls EA, Hoogland G. Gender differences in febrile seizure-induced proliferation and survival in the rat dentate gyrus. Epilepsia. 2005. 46:1603–1612.
Article41. Bender RA, Dubé C, Gonzalez-Vega R, Mina EW, Baram TZ. Mossy fiber plasticity and enhanced hippocampal excitability, without hippocampal cell loss or altered neurogenesis, in an animal model of prolonged febrile seizures. Hippocampus. 2003. 13:399–412.
Article42. Armstrong DD. The neuropathology of temporal lobe epilepsy. J Neuropathol Exp Neurol. 1993. 52:433–443.
Article43. Kasper BS, Stefan H, Paulus W. Microdysgenesis in mesial temporal lobe epilepsy: a clinicopathological study. Ann Neurol. 2003. 54:501–506.
Article44. Diehl B, Najm I, LaPresto E, Prayson R, Ruggieri P, Mohamed A, et al. Temporal lobe volumes in patients with hippocampal sclerosis with or without cortical dysplasia. Neurology. 2004. 62:1729–1735.
Article45. Kalnins RM, McIntosh A, Saling MM, Berkovic SF, Jackson GD, Briellmann RS. Subtle microscopic abnormalities in hippocampal sclerosis do not predict clinical features of temporal lobe epilepsy. Epilepsia. 2004. 45:940–947.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Asymmetry of Medial and Lateral Tempora) Regional Glucose Metabolism in Temporal Lobe Epilepsy by F-18-FDG PET
- Comparison of rCBF between Patients with Medial Temporal Lobe Epilepsy and Normal Controls using H215O PET
- Morphological Alterations of Hippocampus in Temporal Lobe Epilepsy: Cell Loss, Synaptic Reorganization, Cell Birth
- Alice in Wonderland Syndrome in a Case with Infarct in the Right Medial Temporal Lobe
- Temporal lobe epilepsy caused by intrahippocampal calcified cysticercus: a case report