J Breast Cancer.
2012 Dec;15(4):388-392. 10.4048/jbc.2012.15.4.388.
Effect of bFGF on the MCF-7 Cell Cycle with CD44+/CD24-: Promoting the G0/G1-->G2/S Transition
- Affiliations
-
- 1The First Affilated Hospital of Binzhou Medical University, Binzhou, China. yzhlin@126.com
- KMID: 2286434
- DOI: http://doi.org/10.4048/jbc.2012.15.4.388
Abstract
- PURPOSE
Few cells with stem cell characteristics possess capabilities of self-renewal and differentiation, which leads to high tumorigenesis and resistance to standard chemotherapeutic agents. These cells are mostly quiescent, and arrest occurs at the mitotic G0/G1 phase in mitosis. We explored the effects of basic fibroblast growth factor (bFGF) on the MCF-7 cell cycle with CD44+/CD24-.
METHODS
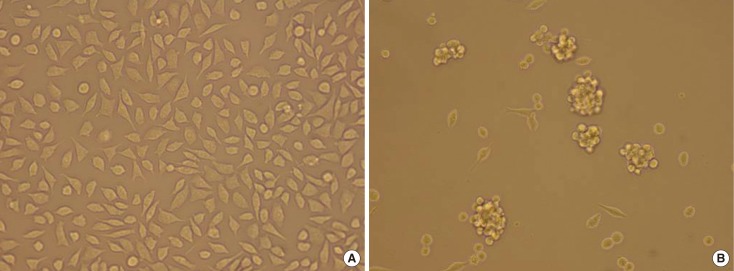
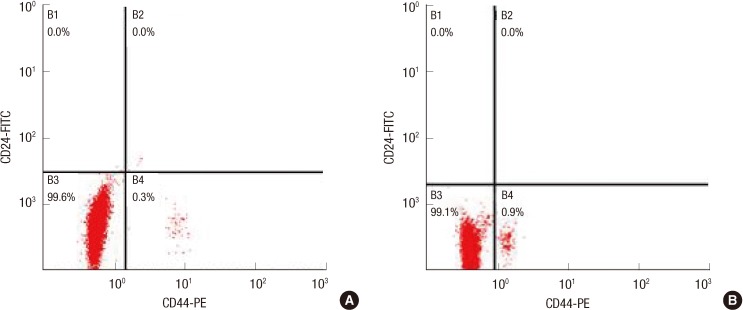
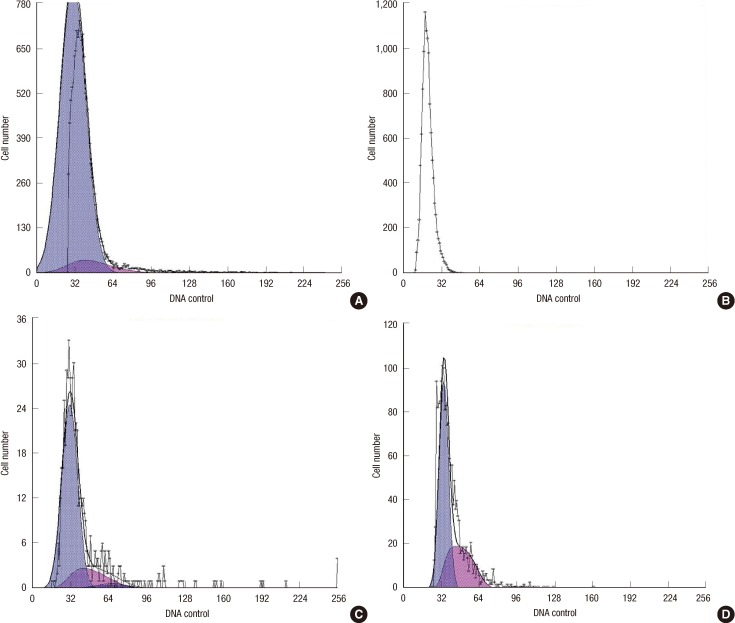
Cancer-initiating cells were propagated as mammospheres. The CD44+/CD24- subpopulation was sorted by a fluorescence activating cell sorter-Vantage flow cytometer. A cell cycle analysis was performed with different bFGF concentrations.
RESULTS
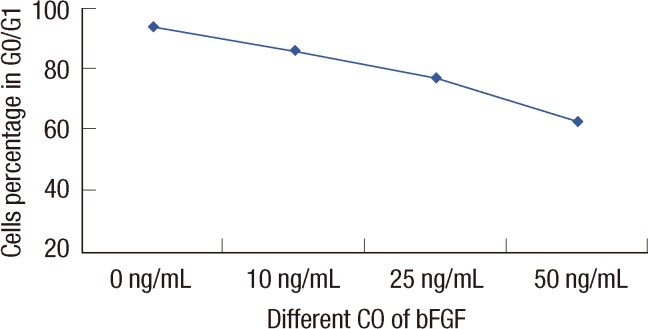
Differences in the CD44+/CD24- cell proliferation under different bFGF concentrations were observed (p=0.001). When the bFGF concentration was increased, the proportion of CD44+/CD24- at G0/G1 decreased (p=0.023).
CONCLUSION
We conclude that bFGF may sustain CD44+/CD24- cell proliferation and could promote cell progression through the G0/G1-->G2/S phase transition.
MeSH Terms
Figure
Reference
-
1. Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003; 3:895–902. PMID: 14737120.
Article2. Bhat-Nakshatri P, Appaiah H, Ballas C, Pick-Franke P, Goulet R Jr, Badve S, et al. SLUG/SNAI2 and tumor necrosis factor generate breast cells with CD44+/CD24- phenotype. BMC Cancer. 2010; 10:411. PMID: 20691079.
Article3. Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008; 100:672–679. PMID: 18445819.
Article4. Phillips TM, McBride WH, Pajonk F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006; 98:1777–1785. PMID: 17179479.5. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003; 100:3983–3988. PMID: 12629218.
Article6. Li HZ, Yi TB, Wu ZY. Suspension culture combined with chemotherapeutic agents for sorting of breast cancer stem cells. BMC Cancer. 2008; 8:135. PMID: 18477410.
Article7. Liu R, Wang X, Chen GY, Dalerba P, Gurney A, Hoey T, et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med. 2007; 356:217–226. PMID: 17229949.
Article8. Gospodarowicz D. Purification of a fibroblast growth factor from bovine pituitary. J Biol Chem. 1975; 250:2515–2520. PMID: 1168187.
Article9. Praul CA, Ford BC, Leach RM. Effect of fibroblast growth factors 1, 2, 4, 5, 6, 7, 8, 9, and 10 on avian chondrocyte proliferation. J Cell Biochem. 2002; 84:359–366. PMID: 11787065.
Article10. Bouche G, Gas N, Prats H, Baldin V, Tauber JP, Teissié J, et al. Basic fibroblast growth factor enters the nucleolus and stimulates the transcription of ribosomal genes in ABAE cells undergoing G0: G1 transition. Proc Natl Acad Sci U S A. 1987; 84:6770–6774. PMID: 3477808.11. Peulve P, Laquerriere A, Paresy M, Hemet J, Tadie M. Establishment of adult rat Schwann cell cultures: effect of b-FGF, alpha-MSH, NGF, PDGF, and TGF-beta on cell cycle. Exp Cell Res. 1994; 214:543–550. PMID: 7925648.12. Frederick TJ, Min J, Altieri SC, Mitchell NE, Wood TL. Synergistic induction of cyclin D1 in oligodendrocyte progenitor cells by IGF-I and FGF-2 requires differential stimulation of multiple signaling pathways. Glia. 2007; 55:1011–1022. PMID: 17508424.
Article13. Takeuchi R, Matsumoto H, Okada H, Hori M, Gunji A, Hakozaki K, et al. Differences of cell growth and cell cycle regulators induced by basic fibroblast growth factor between nifedipine responders and non-responders. J Pharmacol Sci. 2007; 103:168–174. PMID: 17287590.
Article14. Hwang-Verslues WW, Chang KJ, Lee EY, Lee WH. Breast cancer stem cells and tumor suppressor genes. J Formos Med Assoc. 2008; 107:751–766. PMID: 18926942.
Article15. Pratsinis H, Kletsas D. PDGF, bFGF and IGF-I stimulate the proliferation of intervertebral disc cells in vitro via the activation of the ERK and Akt signaling pathways. Eur Spine J. 2007; 16:1858–1866. PMID: 17763874.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Augmentation of Butyrate-induced Differentiation of Human Hepatocyte for the Development of an Efficient Bioartificial Liver
- The effects of gamma-radiation on cyclin-dependent kinases and their inhibitors in cultured vascular smooth muscle cells
- The Changes of Cell Cycle Phase Fractions and Expression of p53 by the Treatment of Staurosporine in MCF-7 Cell Line
- Monolithic zirconia crowns: effect of thickness reduction on fatigue behavior and failure load
- Effects of HMGB-1 Overexpression on Cell-Cycle Progression in MCF-7 Cells