Korean J Physiol Pharmacol.
2015 Mar;19(2):111-118. 10.4196/kjpp.2015.19.2.111.
Long Term Effect of High Glucose and Phosphate Levels on the OPG/RANK/RANKL/TRAIL System in the Progression of Vascular Calcification in rat Aortic Smooth Muscle Cells
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Pusan National University School of Medicine, Yangsan 626-770, Korea. sonsm@pusan.ac.kr
- 2Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan 626-770, Korea.
- 3Center and Endocrine Clinic, Pusan National University Yangsan Hospital, Yangsan 626-770, Korea.
- KMID: 2285562
- DOI: http://doi.org/10.4196/kjpp.2015.19.2.111
Abstract
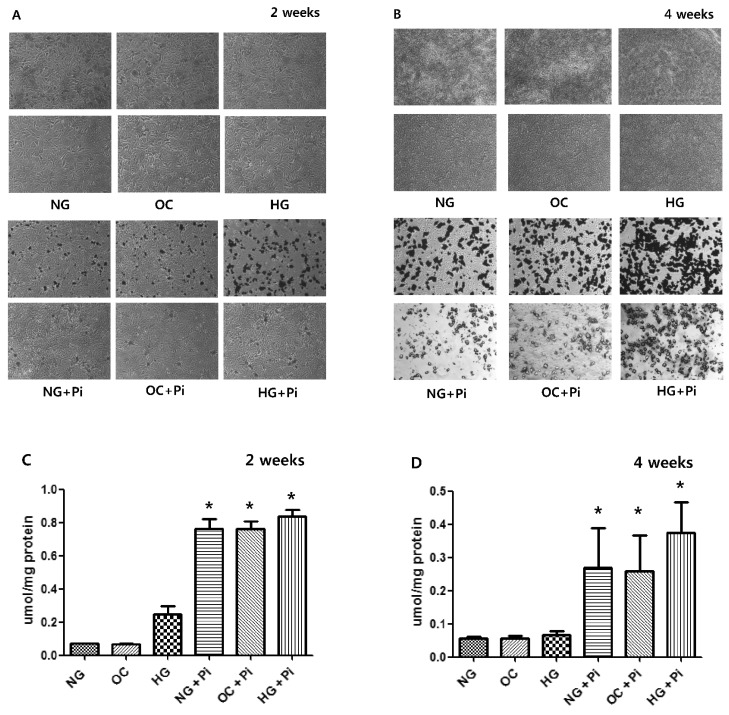
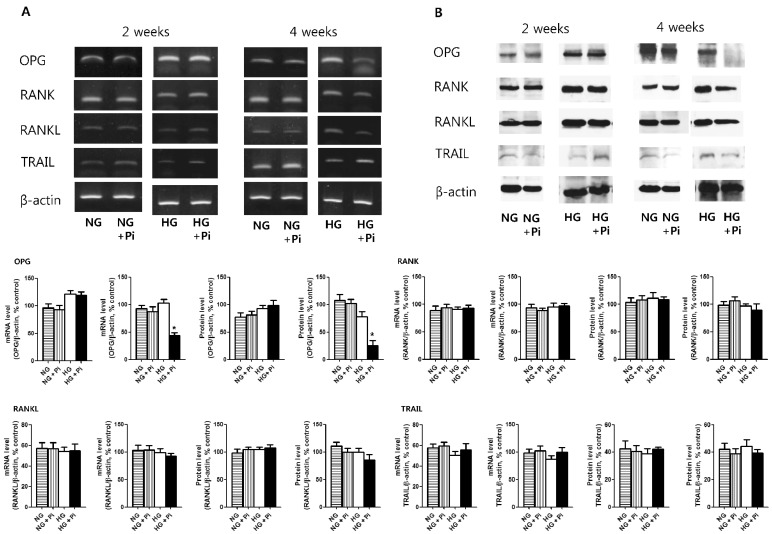
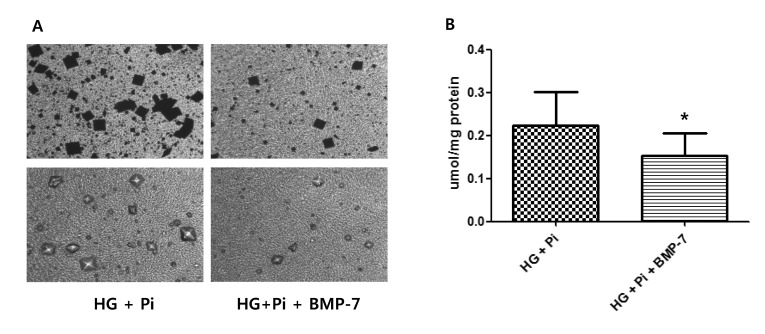
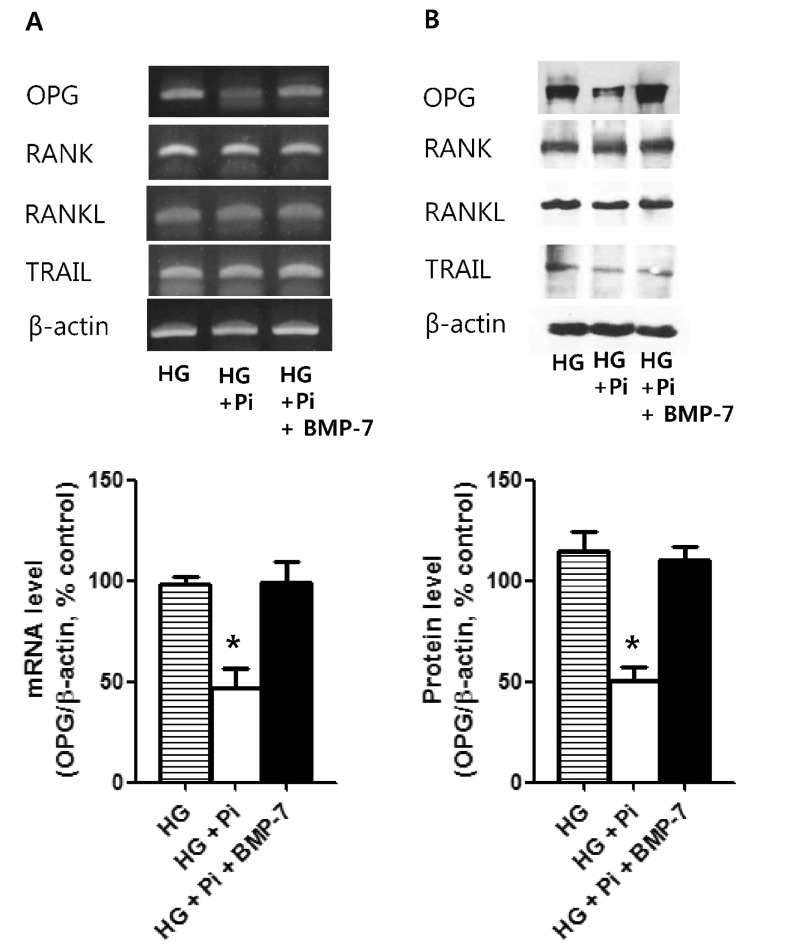
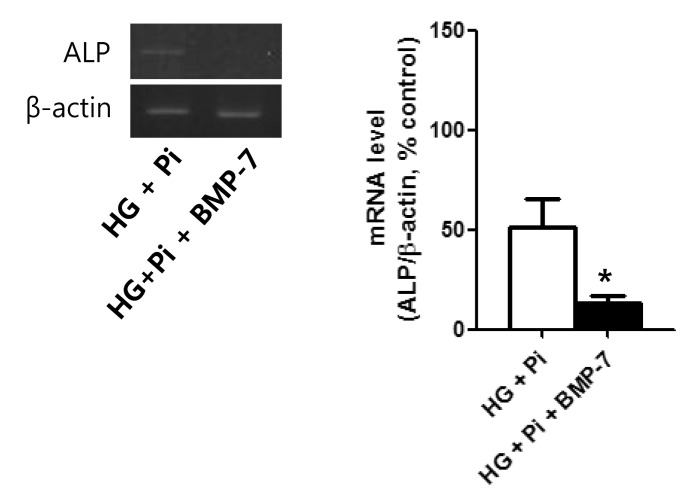
- Osteoprotegerin (OPG), receptor activator of NF-kappaB ligand (RANKL)/receptor activator of NF-kappaB (RANK) axis, and TNF-related apoptosis-inducing ligand (TRAIL) participate in vascular calcification process including atherosclerosis, but their contributions under high glucose (HG) and phosphate (HP) condition for a long-term period (more than 2 weeks) have not been fully determined. In this study, we evaluated the effects of HG and HP levels over 2 or 4 weeks on the progression of vascular calcification in rat vascular smooth muscle cells (VSMCs). Calcium deposition in VSMCs was increased in medium containing HG (30 mmol/L D-glucose) with beta-glycerophosphate (beta-GP, 12 mmol/L) after 2 weeks and increased further after 4 weeks. OPG mRNA and protein expressions were unchanged in HG group with or without beta-GP after 2 weeks. However, after 4 weeks, OPG mRNA and protein expressions were significantly lower in HG group with beta-GP. No significant expression changes were observed in RANKL, RANK, or TRAIL during the experiment. After 4 weeks of treatment in HG group containing beta-GP and rhBMP-7, an inhibitor of vascular calcification, OPG expressions were maintained. Furthermore, mRNA expression of alkaline phosphatase (ALP), a marker of vascular mineralization, was lower in the presence of rhBMP-7. These results suggest that low OPG levels after long term HG and phosphate stimulation might reduce the binding of OPG to RANKL and TRAIL, and these changes could increase osteo-inductive VSMC differentiation, especially vascular mineralization reflected by increased ALP activity during vascular calcification.
Keyword
MeSH Terms
-
Alkaline Phosphatase
Animals
Atherosclerosis
Axis, Cervical Vertebra
Calcium
Glucose*
Muscle, Smooth, Vascular
Myocytes, Smooth Muscle*
NF-kappa B
Osteoprotegerin
Rats*
Receptor Activator of Nuclear Factor-kappa B
RNA, Messenger
TNF-Related Apoptosis-Inducing Ligand
Vascular Calcification*
Alkaline Phosphatase
Calcium
Glucose
NF-kappa B
Osteoprotegerin
RNA, Messenger
Receptor Activator of Nuclear Factor-kappa B
TNF-Related Apoptosis-Inducing Ligand
Figure
Cited by 1 articles
-
CRM1 inhibitor S109 suppresses cell proliferation and induces cell cycle arrest in renal cancer cells
Xuejiao Liu, Yulong Chong, Huize Liu, Yan Han, Mingshan Niu
Korean J Physiol Pharmacol. 2016;20(2):161-168. doi: 10.4196/kjpp.2016.20.2.161.
Reference
-
1. Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006; 99:1044–1059. PMID: 17095733.2. Shioi A, Nishizawa Y, Jono S, Koyama H, Hosoi M, Morii H. Beta-glycerophosphate accelerates calcification in cultured bovine vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1995; 15:2003–2009. PMID: 7583582.3. Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001; 89:1147–1154. PMID: 11739279.4. Chen NX, O'Neill KD, Duan D, Moe SM. Phosphorus and uremic serum up-regulate osteopontin expression in vascular smooth muscle cells. Kidney Int. 2002; 62:1724–1731. PMID: 12371973.
Article5. Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996; 16:978–983. PMID: 8696962.6. Chen NX, Moe SM. Arterial calcification in diabetes. Curr Diab Rep. 2003; 3:28–32. PMID: 12643143.
Article7. Vattikuti R, Towler DA. Osteogenic regulation of vascular calcification: an early perspective. Am J Physiol Endocrinol Metab. 2004; 286:E686–E696. PMID: 15102615.
Article8. Chen NX, Duan D, O'Neill KD, Moe SM. High glucose increases the expression of Cbfa1 and BMP-2 and enhances the calcification of vascular smooth muscle cells. Nephrol Dial Transplant. 2006; 21:3435–3442. PMID: 17005530.
Article9. American Diabetes Association. Standards of medical care in diabetes--2014. Diabetes Care. 2014; 37(Suppl 1):S14–S80. PMID: 24357209.10. Singh VP, Bali A, Singh N, Jaggi AS. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol. 2014; 18:1–14. PMID: 24634591.
Article11. Everhart JE, Pettitt DJ, Knowler WC, Rose FA, Bennett PH. Medial arterial calcification and its association with mortality and complications of diabetes. Diabetologia. 1988; 31:16–23. PMID: 3350219.
Article12. Van Campenhout A, Golledge J. Osteoprotegerin, vascular calcification and atherosclerosis. Atherosclerosis. 2009; 204:321–329. PMID: 19007931.
Article13. Collin-Osdoby P. Regulation of vascular calcification by osteoclast regulatory factors RANKL and osteoprotegerin. Circ Res. 2004; 95:1046–1057. PMID: 15564564.
Article14. Emery JG, McDonnell P, Burke MB, Deen KC, Lyn S, Silverman C, Dul E, Appelbaum ER, Eichman C, DiPrinzio R, Dodds RA, James IE, Rosenberg M, Lee JC, Young PR. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998; 273:14363–14367. PMID: 9603945.
Article15. Gochuico BR, Zhang J, Ma BY, Marshak-Rothstein A, Fine A. TRAIL expression in vascular smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2000; 278:L1045–L1050. PMID: 10781437.
Article16. Kiechl S, Werner P, Knoflach M, Furtner M, Willeit J, Schett G. The osteoprotegerin/RANK/RANKL system: a bone key to vascular disease. Expert Rev Cardiovasc Ther. 2006; 4:801–811. PMID: 17173497.
Article17. Toffoli B, Pickering RJ, Tsorotes D, Wang B, Bernardi S, Kantharidis P, Fabris B, Zauli G, Secchiero P, Thomas MC. Osteoprotegerin promotes vascular fibrosis via a TGF-β1 autocrine loop. Atherosclerosis. 2011; 218:61–68. PMID: 21679949.
Article18. Candido R, Toffoli B, Corallini F, Bernardi S, Zella D, Voltan R, Grill V, Celeghini C, Fabris B. Human full-length osteoprotegerin induces the proliferation of rodent vascular smooth muscle cells both in vitro and in vivo. J Vasc Res. 2010; 47:252–261. PMID: 19907187.19. Olesen P, Ledet T, Rasmussen LM. Arterial osteoprotegerin: increased amounts in diabetes and modifiable synthesis from vascular smooth muscle cells by insulin and TNF-alpha. Diabetologia. 2005; 48:561–568. PMID: 15700136.20. Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA. 2004; 292:490–495. PMID: 15280347.
Article21. Zhang J, Fu M, Myles D, Zhu X, Du J, Cao X, Chen YE. PDGF induces osteoprotegerin expression in vascular smooth muscle cells by multiple signal pathways. FEBS Lett. 2002; 521:180–184. PMID: 12067713.
Article22. Price PA, June HH, Buckley JR, Williamson MK. Osteoprotegerin inhibits artery calcification induced by warfarin and by vitamin D. Arterioscler Thromb Vasc Biol. 2001; 21:1610–1616. PMID: 11597934.
Article23. Min H, Morony S, Sarosi I, Dunstan CR, Capparelli C, Scully S, Van G, Kaufman S, Kostenuik PJ, Lacey DL, Boyle WJ, Simonet WS. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J Exp Med. 2000; 192:463–474. PMID: 10952716.
Article24. Papadopouli AE, Klonaris CN, Theocharis SE. Role of OPG/RANKL/RANK axis on the vasculature. Histol Histopathol. 2008; 23:497–506. PMID: 18228207.25. Davies MR, Lund RJ, Hruska KA. BMP-7 is an efficacious treatment of vascular calcification in a murine model of atherosclerosis and chronic renal failure. J Am Soc Nephrol. 2003; 14:1559–1567. PMID: 12761256.
Article26. Li T, Surendran K, Zawaideh MA, Mathew S, Hruska KA. Bone morphogenetic protein 7: a novel treatment for chronic renal and bone disease. Curr Opin Nephrol Hypertens. 2004; 13:417–422. PMID: 15199292.
Article27. Kang YH, Jin JS, Yi DW, Son SM. Bone morphogenetic protein-7 inhibits vascular calcification induced by high vitamin D in mice. Tohoku J Exp Med. 2010; 221:299–307. PMID: 20647695.
Article28. Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol. 1971; 50:172–186. PMID: 4327464.29. Ha YM, Lee DH, Kim M, Kang YJ. High glucose induces connective tissue growth factor expression and extracellular matrix accumulation in rat aorta vascular smooth muscle cells via extracellular signal-regulated kinase 1/2. Korean J Physiol Pharmacol. 2013; 17:307–314. PMID: 23946690.
Article30. Dorai H, Vukicevic S, Sampath TK. Bone morphogenetic protein-7 (osteogenic protein-1) inhibits smooth muscle cell proliferation and stimulates the expression of markers that are characteristic of SMC phenotype in vitro. J Cell Physiol. 2000; 184:37–45. PMID: 10825232.
Article31. Murshed M, Schinke T, McKee MD, Karsenty G. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J Cell Biol. 2004; 165:625–630. PMID: 15184399.
Article32. Kawashima N, Suzuki N, Yang G, Ohi C, Okuhara S, Nakano-Kawanishi H, Suda H. Kinetics of RANKL, RANK and OPG expressions in experimentally induced rat periapical lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 103:707–711. PMID: 17336108.
Article33. Corallini F, Celeghini C, Rizzardi C, Pandolfi A, Di Silvestre S, Vaccarezza M, Zauli G. Insulin down-regulates TRAIL expression in vascular smooth muscle cells both in vivo and in vitro. J Cell Physiol. 2007; 212:89–95. PMID: 17352408.
Article34. Shioi A, Katagi M, Okuno Y, Mori K, Jono S, Koyama H, Nishizawa Y. Induction of bone-type alkaline phosphatase in human vascular smooth muscle cells: roles of tumor necrosis factor-alpha and oncostatin M derived from macrophages. Circ Res. 2002; 91:9–16. PMID: 12114316.35. Boström KI. Cell differentiation in vascular calcification. Z Kardiol. 2000; 89(Suppl 2):69–74. PMID: 10769406.
Article36. Iyemere VP, Proudfoot D, Weissberg PL, Shanahan CM. Vascular smooth muscle cell phenotypic plasticity and the regulation of vascular calcification. J Intern Med. 2006; 260:192–210. PMID: 16918817.
Article37. Schoppet M, Al-Fakhri N, Franke FE, Katz N, Barth PJ, Maisch B, Preissner KT, Hofbauer LC. Localization of osteoprotegerin, tumor necrosis factor-related apoptosis-inducing ligand, and receptor activator of nuclear factor-kappaB ligand in Mönckeberg's sclerosis and atherosclerosis. J Clin Endocrinol Metab. 2004; 89:4104–4112. PMID: 15292354.38. Al-Fakhri N, Hofbauer LC, Preissner KT, Franke FE, Schoppet M. Expression of bone-regulating factors osteoprotegerin (OPG) and receptor activator of NF-kappaB ligand (RANKL) in heterotopic vascular ossification. Thromb Haemost. 2005; 94:1335–1337. PMID: 16411417.39. Rasmussen LM, Tarnow L, Hansen TK, Parving HH, Flyvbjerg A. Plasma osteoprotegerin levels are associated with glycaemic status, systolic blood pressure, kidney function and cardiovascular morbidity in type 1 diabetic patients. Eur J Endocrinol. 2006; 154:75–81. PMID: 16381994.
Article40. Knudsen ST, Foss CH, Poulsen PL, Andersen NH, Mogensen CE, Rasmussen LM. Increased plasma concentrations of osteoprotegerin in type 2 diabetic patients with microvascular complications. Eur J Endocrinol. 2003; 149:39–42. PMID: 12824864.
Article41. Avignon A, Sultan A, Piot C, Elaerts S, Cristol JP, Dupuy AM. Osteoprotegerin is associated with silent coronary artery disease in high-risk but asymptomatic type 2 diabetic patients. Diabetes Care. 2005; 28:2176–2180. PMID: 16123486.
Article42. Secchiero P, Corallini F, Pandolfi A, Consoli A, Candido R, Fabris B, Celeghini C, Capitani S, Zauli G. An increased osteoprotegerin serum release characterizes the early onset of diabetes mellitus and may contribute to endothelial cell dysfunction. Am J Pathol. 2006; 169:2236–2244. PMID: 17148684.
Article43. Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM, Weissberg PL. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ Res. 2000; 87:1055–1062. PMID: 11090552.44. Hui M, Tenenbaum HC. New face of an old enzyme: alkaline phosphatase may contribute to human tissue aging by inducing tissue hardening and calcification. Anat Rec. 1998; 253:91–94. PMID: 9700394.
Article45. Shanahan CM, Proudfoot D, Tyson KL, Cary NR, Edmonds M, Weissberg PL. Expression of mineralisation-regulating proteins in association with human vascular calcification. Z Kardiol. 2000; 89(Suppl 2):63–68. PMID: 10769405.
Article46. Orita Y, Yamamoto H, Kohno N, Sugihara M, Honda H, Kawamata S, Mito S, Soe NN, Yoshizumi M. Role of osteoprotegerin in arterial calcification: development of new animal model. Arterioscler Thromb Vasc Biol. 2007; 27:2058–2064. PMID: 17615383.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of zinc for calcification inhibitor protein in vascular smooth muscle cell plaque formation
- Synovial RANKL/OPG mRNA Ratio and Effect of IL-17 in Experimental Rheumatoid Arthritis Model
- Osteoprotegerin and Osteoprotegerin Ligand Expression in the Periprosthetic Tissue of Failed Hip Prosthesis
- The effect of cortical punching on the expression of OPG, RANK, and RANKL in the periodontal tissue during tooth movement in rats
- Intraarticular Corticosteroids Modulate the Osteoprotegerin/Receptor Activator of Nuclear Factor-kappaB Ligand System in the Synovial Fluid in Patients with Rheumatoid Arthritis