Korean J Physiol Pharmacol.
2014 Jun;18(3):255-261. 10.4196/kjpp.2014.18.3.255.
The Stimulatory Effect of Essential Fatty Acids on Glucose Uptake Involves Both Akt and AMPK Activation in C2C12 Skeletal Muscle Cells
- Affiliations
-
- 1Department of Physiology, College of Medicine, Hallym University, Chuncheon 200-702, Korea. hykwon@hallym.ac.kr
- 2Department of Pharmacology, College of Medicine, Hallym University, Chuncheon 200-702, Korea.
- 3Department of Biomedical Science and Research Institute of Bioscience and Biotechnology, Hallym University, Chuncheon 200-702, Korea.
- KMID: 2285518
- DOI: http://doi.org/10.4196/kjpp.2014.18.3.255
Abstract
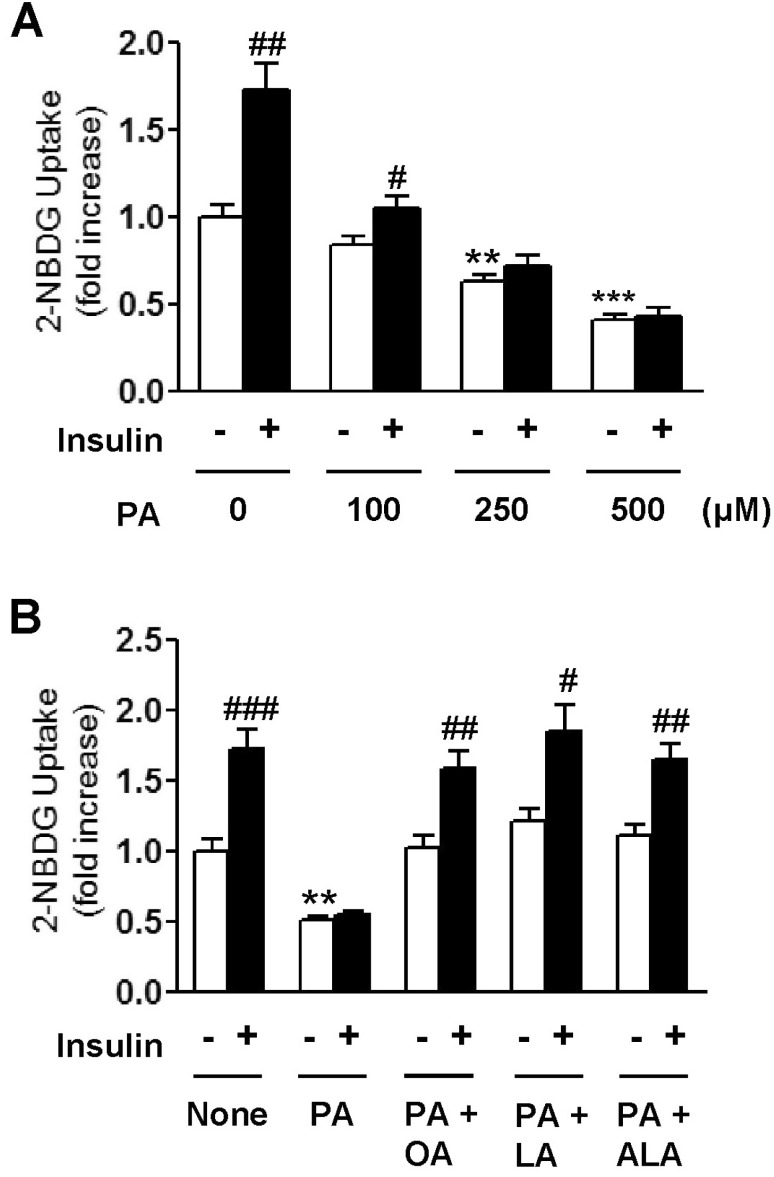
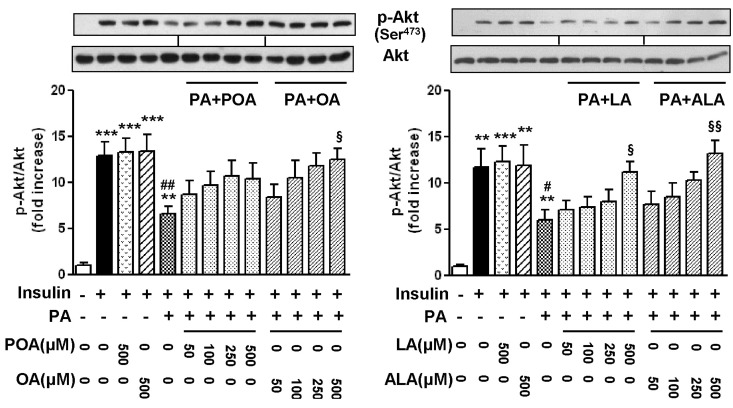
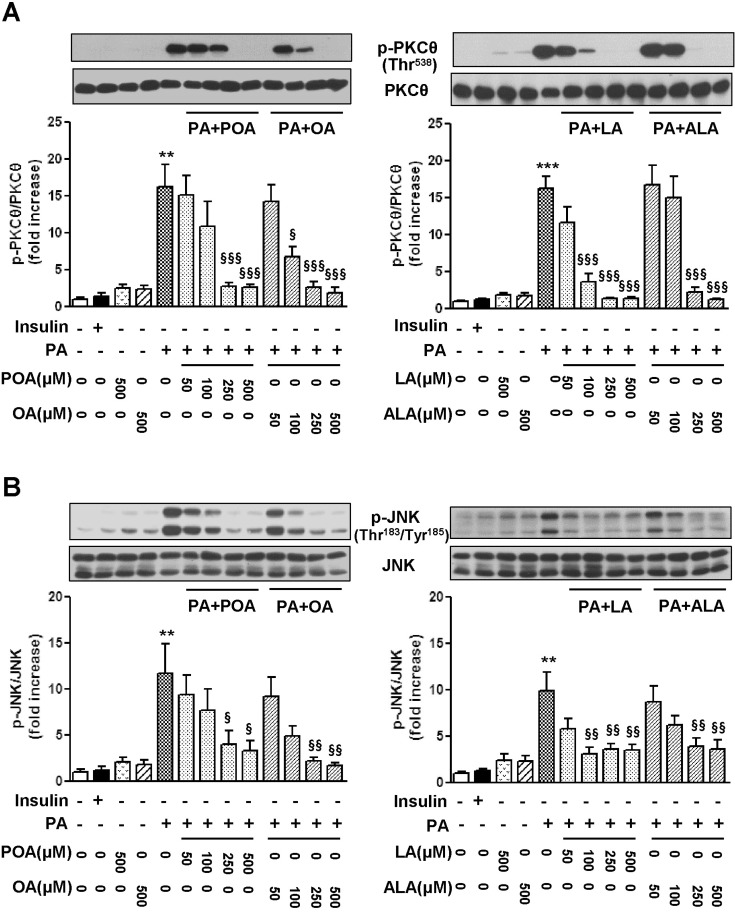
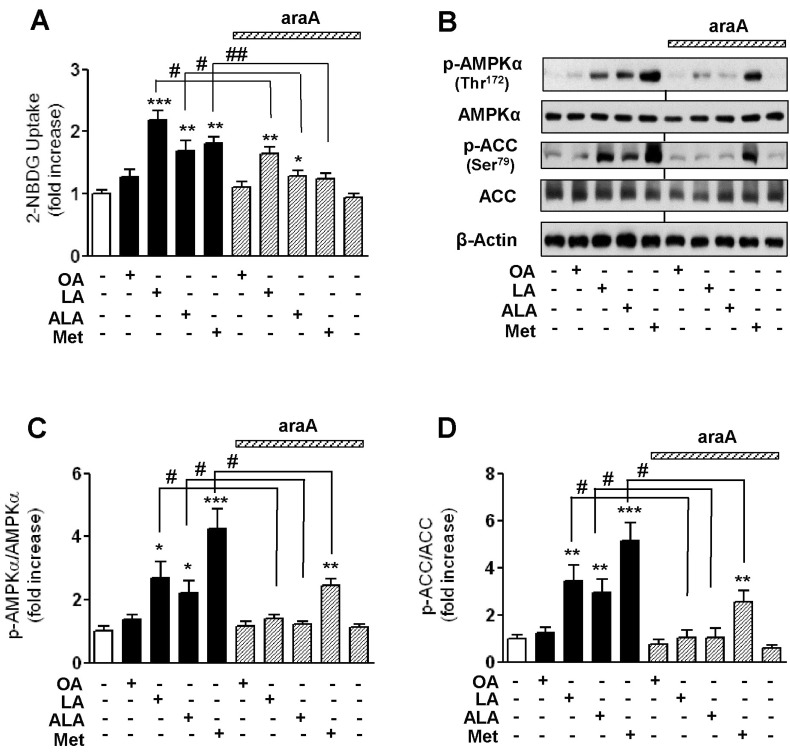
- Essential fatty acid (EFA) is known to be required for the body to function normally and healthily. However, the effect of EFA on glucose uptake in skeletal muscle has not yet been fully investigated. In this study, we examined the effect of two EFAs, linoleic acid (LA) and alpha-linolenic acid (ALA), on glucose uptake of C2C12 skeletal muscle cells and investigated the mechanism underlying the stimulatory effect of polyunsaturated EFAs in comparison with monounsaturated oleic acid (OA). In palmitic acid (PA)-induced insulin resistant cells, the co-treatment of EFAs and OA with PA almost restored the PA-induced decrease in the basal and insulin-stimulated 2-NBDG (fluorescent D-glucose analogue) uptake, respectively. Two EFAs and OA significantly protected PA-induced suppression of insulin signaling, respectively, which was confirmed by the increased levels of Akt phosphorylation and serine/threonine kinases (PKCtheta and JNK) dephosphorylation in the western blot analysis. In PA-untreated, control cells, the treatment of 500 microM EFA significantly stimulated 2-NBDG uptake, whereas OA did not. Phosphorylation of AMP-activated protein kinase (AMPK) and one of its downstream molecules, acetyl-CoA carboxylase (ACC) was markedly induced by EFA, but not OA. In addition, EFA-stimulated 2-NBDG uptake was significantly inhibited by the pre-treatment of a specific AMPK inhibitor, adenine 9-beta-D-arabinofuranoside (araA). These data suggest that the restoration of suppressed insulin signaling at PA-induced insulin resistant condition and AMPK activation are involved at least in the stimulatory effect of EFA on glucose uptake in C2C12 skeletal muscle cells.
MeSH Terms
-
Acetyl-CoA Carboxylase
Adenine
alpha-Linolenic Acid
AMP-Activated Protein Kinases*
Blotting, Western
Fatty Acids, Essential*
Glucose*
Insulin
Linoleic Acid
Muscle, Skeletal*
Oleic Acid
Palmitic Acid
Phosphorylation
Phosphotransferases
AMP-Activated Protein Kinases
Acetyl-CoA Carboxylase
Adenine
Fatty Acids, Essential
Glucose
Insulin
Linoleic Acid
Oleic Acid
Palmitic Acid
Phosphotransferases
alpha-Linolenic Acid
Figure
Cited by 2 articles
-
EGCG Blocked Phenylephrin-Induced Hypertrophy in H9C2 Cardiomyocytes, by Activating AMPK-Dependent Pathway
Yi Cai, Li Zhao, Yuan Qin, Xiao-Qian Wu
Korean J Physiol Pharmacol. 2015;19(3):203-210. doi: 10.4196/kjpp.2015.19.3.203.Identification of AMPK activator from twelve pure compounds isolated from
Aralia Taibaiensis : implication in antihyperglycemic and hypolipidemic activities
Yuwen Li, Jongsun Park, Yin Wu, Jia Cui, Na Jia, Miaomiao Xi, Aidong Wen
Korean J Physiol Pharmacol. 2017;21(3):279-286. doi: 10.4196/kjpp.2017.21.3.279.
Reference
-
1. Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006; 7:85–96. PMID: 16493415.
Article2. Thirone AC, Huang C, Klip A. Tissue-specific roles of IRS proteins in insulin signaling and glucose transport. Trends Endocrinol Metab. 2006; 17:72–78. PMID: 16458527.
Article3. Gual P, Le Marchand-Brustel Y, Tanti JF. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie. 2005; 87:99–109. PMID: 15733744.
Article4. Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002; 277:1531–1537. PMID: 11606564.
Article5. Coll T, Eyre E, Rodríguez-Calvo R, Palomer X, Sánchez RM, Merlos M, Laguna JC, Vázquez-Carrera M. Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J Biol Chem. 2008; 283:11107–11116. PMID: 18281277.
Article6. Turpin SM, Lancaster GI, Darby I, Febbraio MA, Watt MJ. Apoptosis in skeletal muscle myotubes is induced by ceramides and is positively related to insulin resistance. Am J Physiol Endocrinol Metab. 2006; 291:E1341–E1350. PMID: 16849630.
Article7. Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008; 118:2992–3002. PMID: 18769626.
Article8. Gao D, Griffiths HR, Bailey CJ. Oleate protects against palmitate-induced insulin resistance in L6 myotubes. Br J Nutr. 2009; 102:1557–1563. PMID: 19622194.
Article9. Yuzefovych L, Wilson G, Rachek L. Different effects of oleate vs. palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells: role of oxidative stress. Am J Physiol Endocrinol Metab. 2010; 299:E1096–E1105. PMID: 20876761.
Article10. Ahn JH, Kim MH, Kwon HJ, Choi SY, Kwon HY. Protective Effects of Oleic Acid Against Palmitic Acid-Induced Apoptosis in Pancreatic AR42J Cells and Its Mechanisms. Korean J Physiol Pharmacol. 2013; 17:43–50. PMID: 23440052.
Article11. Hardie DG. The AMP-activated protein kinase pathway--new players upstream and downstream. J Cell Sci. 2004; 117:5479–5487. PMID: 15509864.12. Viollet B, Mounier R, Leclerc J, Yazigi A, Foretz M, Andreelli F. Targeting AMP-activated protein kinase as a novel therapeutic approach for the treatment of metabolic disorders. Diabetes Metab. 2007; 33:395–402. PMID: 17997341.13. Ha J, Daniel S, Broyles SS, Kim KH. Critical phosphorylation sites for acetyl-CoA carboxylase activity. J Biol Chem. 1994; 269:22162–22168. PMID: 7915280.
Article14. Reiffel JA, McDonald A. Antiarrhythmic effects of omega-3 fatty acids. Am J Cardiol. 2006; 98:50i–60i.
Article15. Kruger MC, Horrobin DF. Calcium metabolism, osteoporosis and essential fatty acids: a review. Prog Lipid Res. 1997; 36:131–151. PMID: 9624425.16. Sawada K, Kawabata K, Yamashita T, Kawasaki K, Yamamoto N, Ashida H. Ameliorative effects of polyunsaturated fatty acids against palmitic acid-induced insulin resistance in L6 skeletal muscle cells. Lipids Health Dis. 2012; 11:36–44. PMID: 22409911.
Article17. Qin H, Liu Y, Lu N, Li Y, Sun CH. cis-9,trans-11-Conjugated linoleic acid activates AMP-activated protein kinase in attenuation of insulin resistance in C2C12 myotubes. J Agric Food Chem. 2009; 57:4452–4458. PMID: 19364109.
Article18. Mohankumar SK, Taylor CG, Siemens L, Zahradka P. Activation of phosphatidylinositol-3 kinase, AMP-activated kinase and Akt substrate-160 kDa by trans-10, cis-12 conjugated linoleic acid mediates skeletal muscle glucose uptake. J Nutr Biochem. 2013; 24:445–456. PMID: 22704782.
Article19. Mohankumar SK, Taylor CG, Siemens L, Zahradka P. Acute exposure of L6 myotubes to cis-9, trans-11 and trans-10, cis-12 conjugated linoleic acid isomers stimulates glucose uptake by modulating Ca2+/calmodulin-dependent protein kinase II. Int J Biochem Cell Biol. 2012; 44:1321–1330. PMID: 22609102.20. Spector AA. Fatty acid binding to plasma albumin. J Lipid Res. 1975; 16:165–179. PMID: 236351.
Article21. Zou C, Wang Y, Shen Z. 2-NBDG as a fluorescent indicator for direct glucose uptake measurement. J Biochem Biophys Methods. 2005; 64:207–215. PMID: 16182371.
Article22. Deng YT, Chang TW, Lee MS, Lin JK. Suppression of free fatty acid-induced insulin resistance by phytopolyphenols in C2C12 mouse skeletal muscle cells. J Agric Food Chem. 2012; 60:1059–1066. PMID: 22191431.
Article23. Kim YJ, Kim EA, Chung ML, Im C. Cytotoxic Activity and three-dimensional quantitative structure activity relationship of 2-Aryl-1,8-naphthyridin-4-ones. Korean J Physiol Pharmacol. 2009; 13:511–516. PMID: 20054500.
Article24. Jung JG, Choi SE, Hwang YJ, Lee SA, Kim EK, Lee MS, Han SJ, Kim HJ, Kim DJ, Kang Y, Lee KW. Supplementation of pyruvate prevents palmitate-induced impairment of glucose uptake in C2 myotubes. Mol Cell Endocrinol. 2011; 345:79–87. PMID: 21802492.
Article25. Yang C, Aye CC, Li X, Diaz Ramos A, Zorzano A, Mora S. Mitochondrial dysfunction in insulin resistance: differential contributions of chronic insulin and saturated fatty acid exposure in muscle cells. Biosci Rep. 2012; 32:465–478. PMID: 22742515.
Article26. Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic S, Moller DE, Thorell A, Goodyear LJ. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002; 51:2074–2081. PMID: 12086935.
Article27. Salvadó L, Coll T, Gómez-Foix AM, Salmerón E, Barroso E, Palomer X, Vázquez-Carrera M. Oleate prevents saturated-fatty-acid-induced ER stress, inflammation and insulin resistance in skeletal muscle cells through an AMPK-dependent mechanism. Diabetologia. 2013; 56:1372–1382. PMID: 23460021.
Article28. Musi N, Hayashi T, Fujii N, Hirshman MF, Witters LA, Goodyear LJ. AMP-activated protein kinase activity and glucose uptake in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2001; 280:E677–E684. PMID: 11287349.
Article29. Wu X, Motoshima H, Mahadev K, Stalker TJ, Scalia R, Goldstein BJ. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes. 2003; 52:1355–1363. PMID: 12765944.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- AMPK and Exercise: Glucose Uptake and Insulin Sensitivity
- Resveratrol stimulates glucose transport in C2C12 myotubes by activating AMP-activated protein kinase
- The effects of Allomyrina dichotoma larval extract on palmitate-induced insulin resistance in skeletal muscle cells
- 17Beta-estradiol Stimulates Glucose Uptake Through Estrogen Receptor and AMP-activated Protein Kinase Activation in C2C12 Myotubes
- Increase in Fatty Acid Oxidation by AICAR: the Role of p38 MAPK