Korean J Physiol Pharmacol.
2014 Feb;18(1):41-46. 10.4196/kjpp.2014.18.1.41.
The Modulatory Role of Spinally Located Histamine Receptors in the Regulation of the Blood Glucose Level in D-Glucose-Fed Mice
- Affiliations
-
- 1Department of Pharmacology, Institute of Natural Medicine, College of Medicine, Hallym University, Chuncheon 200-702, Korea. hwsuh@hallym.ac.kr
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, College of Medicine, Hallym University, Chuncheon 200-702, Korea.
- KMID: 2285489
- DOI: http://doi.org/10.4196/kjpp.2014.18.1.41
Abstract
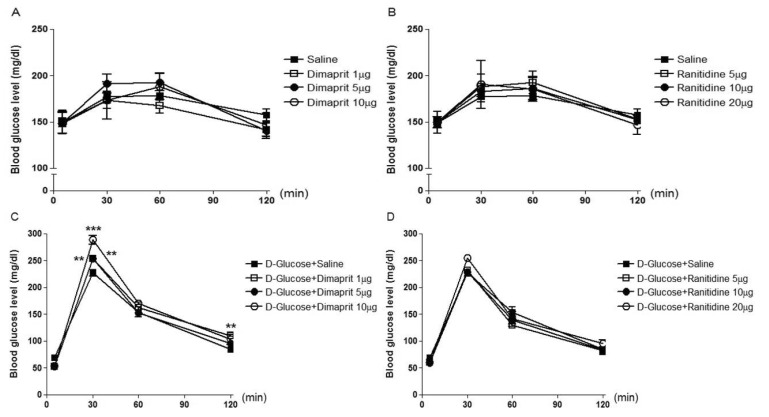
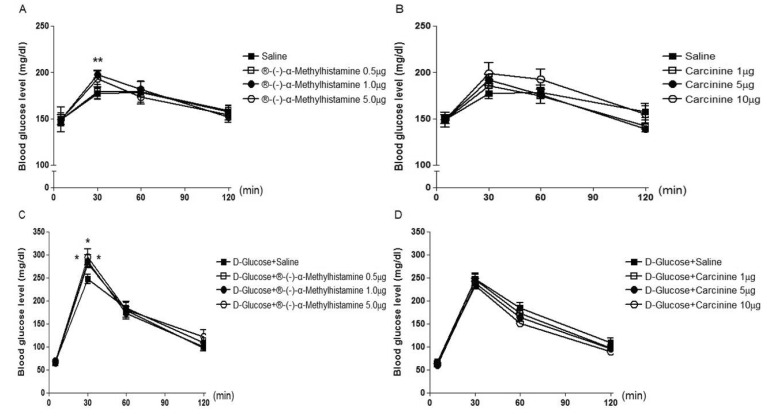
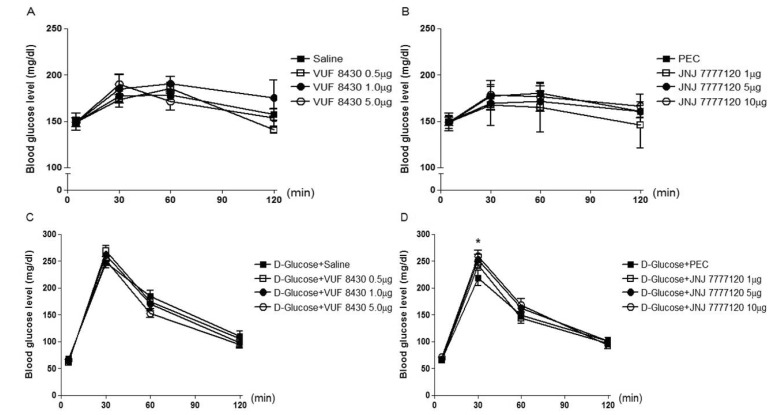
- The possible roles of spinal histamine receptors in the regulation of the blood glucose level were studied in ICR mice. Mice were intrathecally (i.t.) treated with histamine 1 (H1) receptor agonist (2-pyridylethylamine) or antagonist (cetirizine), histamine 2 (H2) receptor agonist (dimaprit) or antagonist (ranitidine), histamine 3 (H3) receptor agonist (alpha-methylhistamine) or antagonist (carcinine) and histamine 4 (H4) receptor agonist (VUF 8430) or antagonist (JNJ 7777120), and the blood glucose level was measured at 30, 60 and 120 min after i.t. administration. The i.t. injection with alpha-methylhistamine, but not carcinine slightly caused an elevation of the blood glucose level. In addition, histamine H1, H2, and H4 receptor agonists and antagonists did not affect the blood glucose level. In D-glucose-fed model, i.t. pretreatment with cetirizine enhanced the blood glucose level, whereas 2-pyridylethylamine did not affect. The i.t. pretreatment with dimaprit, but not ranitidine, enhanced the blood glucose level in D-glucose-fed model. In addition, alpha-methylhistamine, but not carcinine, slightly but significantly enhanced the blood glucose level D-glucose-fed model. Finally, i.t. pretreatment with JNJ 7777120, but not VUF 8430, slightly but significantly increased the blood glucose level. Although histamine receptors themselves located at the spinal cord do not exert any effect on the regulation of the blood glucose level, our results suggest that the activation of spinal histamine H2 receptors and the blockade of spinal histamine H1 or H3 receptors may play modulatory roles for up-regulation and down-regulation, respectively, of the blood glucose level in D-glucose fed model.
Keyword
MeSH Terms
-
Animals
Blood Glucose*
Cetirizine
Dimaprit
Down-Regulation
Glucose
Histamine*
Mice*
Mice, Inbred ICR
Ranitidine
Receptors, Histamine H2
Receptors, Histamine H3
Receptors, Histamine*
Spinal Cord
Up-Regulation
Blood Glucose
Cetirizine
Dimaprit
Glucose
Histamine
Ranitidine
Receptors, Histamine
Receptors, Histamine H2
Receptors, Histamine H3
Figure
Reference
-
1. Inoue I, Yanai K, Kitamura D, Taniuchi I, Kobayashi T, Niimura K, Watanabe T, Watanabe T. Impaired locomotor activity and exploratory behavior in mice lacking histamine H1 receptors. Proc Natl Acad Sci U S A. 1996; 93:13316–13320. PMID: 8917588.
Article2. Tashiro M, Mochizuki H, Iwabuchi K, Sakurada Y, Itoh M, Watanabe T, Yanai K. Roles of histamine in regulation of arousal and cognition: functional neuroimaging of histamine H1 receptors in human brain. Life Sci. 2002; 72:409–414. PMID: 12467881.
Article3. Stacpoole PW, Robertson D. Combination H1 and H2 receptor antagonist therapy in diabetic autonomic neuropathy. South Med J. 1982; 75:634–635. PMID: 6123154.
Article4. Schusdziarra V, Rouiller D, Harris V, Pfeiffer EF, Unger RH. Role of H2-receptors in gastrogenic hyperglycemia and hyperinsulinemia in dogs. Regul Pept. 1982; 3:245–250. PMID: 7043663.
Article5. Lal A. Effect of a few histamine(1)-antagonists on blood glucose in patients of allergic rhinitis. Indian J Otolaryngol Head Neck Surg. 2000; 52:193–195. PMID: 23119674.
Article6. Brackett DJ, Schaefer CF, Wilson MF. The effects of H1 and H2 histamine receptor antagonists on the development of endotoxemia in the conscious, unrestrained rat. Circ Shock. 1985; 16:141–153. PMID: 2414028.7. Grund VR, Martino R, Hunninghake DB. Cimetidine blockade of histamine-induced insulin secretion. Clin Pharmacol Ther. 1980; 28:392–397. PMID: 6996897.
Article8. Nagai K, Frohman LA. Neurotensin hyperglycemia:evidence for histamine mediation and the assessment of a possible physiologic role. Diabetes. 1978; 27:577–582. PMID: 648748.
Article9. Nonogaki K, Iguchi A, Li X, Tamagawa T, Watanabe G, Hiyoshi Y, Sakamoto N. Role of brain histamine H1- and H2-receptors in neostigmine-induced hyperglycemia in rats. Life Sci. 1992; 51:PL131–PL134. PMID: 1518366.
Article10. Feely J, Collins WC, Cullen M, el Debani AH, MacWalter RS, Peden NR, Stevenson IH. Potentiation of the hypoglycaemic response to glipizide in diabetic patients by histamine H2-receptor antagonists. Br J Clin Pharmacol. 1993; 35:321–323. PMID: 8471413.
Article11. Czyzyk A, Lao B, Szutowski M, Szczepanik Z, Muszyński J. Enhancement of alcohol-induced hypoglycaemia by H2-receptor antagonists. Arzneimittelforschung. 1997; 47:746–749. PMID: 9239453.12. Scarpignato C, Tirelli F, Starcich R, Bertaccini G. Effect of acute and chronic cimetidine administration on glucose tolerance and insulin secretion in man. Horm Res. 1981; 15:228–236. PMID: 6765588.
Article13. Zacny E, Bugajski J. The effect of clonidine injected centrally on serum-free fatty acids and glucose concentration in the rat. Metabolism. 1983; 32:938–942. PMID: 6888274.
Article14. DiTullio NW, Cieslinski L, Matthews WD, Storer B. Mechanisms involved in the hyperglycemic response induced by clonidine and other alpha-2 adrenoceptor agonists. J Pharmacol Exp Ther. 1984; 228:168–173. PMID: 6141276.15. Nishibori M, Itoh Y, Oishi R, Saeki K. Mechanism of the central hyperglycemic action of histamine in mice. J Pharmacol Exp Ther. 1987; 241:582–586. PMID: 3572814.16. Hylden JL, Wilcox GL. Intrathecal substance P elicits a caudally-directed biting and scratching behavior in mice. Brain Res. 1981; 217:212–215. PMID: 6167328.
Article17. Hancock AA, Bennani YL, Bush EN, Esbenshade TA, Faghih R, Fox GB, Jacobson P, Knourek-Segel V, Krueger KM, Nuss ME, Pan JB, Shapiro R, Witte DG, Yao BB. Antiobesity effects of A-331440, a novel non-imidazole histamine H3 receptor antagonist. Eur J Pharmacol. 2004; 487:183–197. PMID: 15033391.
Article18. Cannon KE, Chazot PL, Hann V, Shenton F, Hough LB, Rice FL. Immunohistochemical localization of histamine H3 receptors in rodent skin, dorsal root ganglia, superior cervical ganglia, and spinal cord: potential antinociceptive targets. Pain. 2007; 129:76–92. PMID: 17134835.
Article19. Taylor JE, Yaksh TL, Richelson E. Histamine H1 receptors in the brain and spinal cord of the cat. Brain Res. 1982; 243:391–394. PMID: 7104749.
Article20. Lethbridge NL, Chazot PL. Immunological identification of the mouse H4 histamine receptor on spinal cord motor neurons using a novel anti-mouse H4R antibody. Inflamm Res. 2010; 59(Suppl 2):S197–S198. PMID: 20020316.
Article21. Wu GY, Han XH, Zhuang QX, Zhang J, Yung WH, Chan YS, Zhu JN, Wang JJ. Excitatory effect of histamine on rat spinal motoneurons by activation of both H1 and H2 receptors in vitro. J Neurosci Res. 2012; 90:132–142. PMID: 21922515.22. Henry MB, Zheng S, Duan C, Patel B, Vassileva G, Sondey C, Lachowicz J, Hwa JJ. Antidiabetic properties of the histamine H3 receptor protean agonist proxyfan. Endocrinology. 2011; 152:828–835. PMID: 21239440.
Article23. Hasanein P. Effects of histamine H3 receptors on chemical hyperalgesia in diabetic rats. Neuropharmacology. 2011; 60:886–891. PMID: 21237180.
Article24. Sala F, Menna G, Bricolo A, Young W. Role of glycemia in acute spinal cord injury. Data from a rat experimental model and clinical experience. Ann N Y Acad Sci. 1999; 890:133–154. PMID: 10668421.
Article25. Sim YB, Park SH, Kang YJ, Jung JS, Ryu OH, Choi MG, Suh HW. Interleukin-1β (IL-1β) increases pain behavior and the blood glucose level: possible involvement of sympathetic nervous system. Pharmacol Biochem Behav. 2012; 102:170–176. PMID: 22548833.
Article26. Sim YB, Park SH, Kang YJ, Jung JS, Ryu OH, Choi MG, Suh HW. Various pain stimulations cause an increase of the blood glucose level. Animal Cells Syst. 2012; 16:385–390.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Cholera Toxin Administered Supraspinally or Spinally on the Blood Glucose Level in Pain and D-Glucose Fed Animal Models
- Intracerebroventricular Kainic Acid-Induced Damage Affects Blood Glucose Level in d-glucose-fed Mouse Model
- Repaglinide, but Not Nateglinide Administered Supraspinally and Spinally Exerts an Anti-Diabetic Action in D-Glucose Fed and Streptozotocin-Treated Mouse Models
- Role of Pyruvate Dehydrogenase Kinase 4 in Regulation of Blood Glucose Levels
- Effect of D-glucose feeding on mortality induced by sepsis