J Adv Prosthodont.
2014 Dec;6(6):539-546. 10.4047/jap.2014.6.6.539.
Comparable efficacy of silk fibroin with the collagen membranes for guided bone regeneration in rat calvarial defects
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, Hallym University School of Medicine, Hallym University Sacred Heart Hospital, Anyang, Republic of Korea.
- 2Department of Prosthodontics, Hallym University School of Medicine, Hallym University Sacred Heart Hospital, Anyang, Republic of Korea. hyewonshim@hallym.or.kr
- 3Department of Emergency Medicine, Konkuk University School of medicine, Konkuk University Medical Center, Seoul, Republic of Korea.
- KMID: 2284729
- DOI: http://doi.org/10.4047/jap.2014.6.6.539
Abstract
- PURPOSE
Silk fibroin (SF) is a new degradable barrier membrane for guided bone regeneration (GBR) that can reduce the risk of pathogen transmission and the high costs associated with the use of collagen membranes. This study compared the efficacy of SF membranes on GBR with collagen membranes (Bio-Gide(R)) using a rat calvarial defect model.
MATERIALS AND METHODS
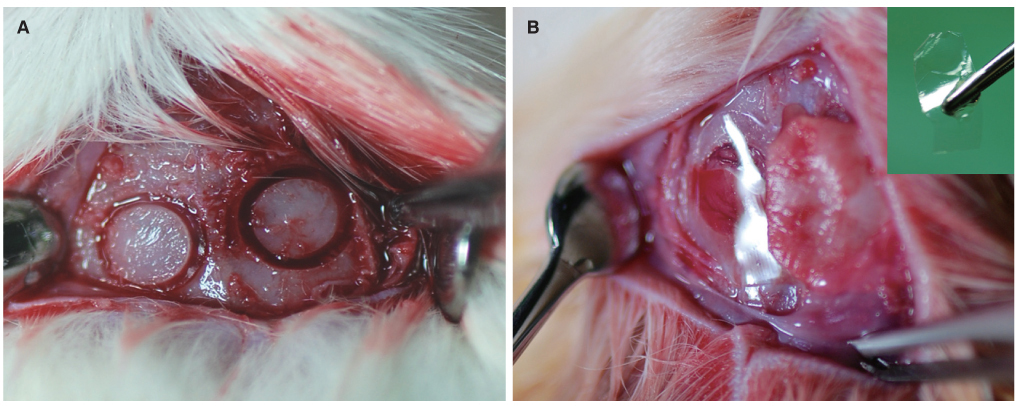
Thirty-six male Sprague Dawley rats with two 5 mm-sized circular defects in the calvarial bone were prepared (n=72). The study groups were divided into a control group (no membrane) and two experimental groups (SF membrane and Bio-Gide(R)). Each group of 24 samples was subdivided at 2, 4, and 8 weeks after implantation. New bone formation was evaluated using microcomputerized tomography and histological examination.
RESULTS
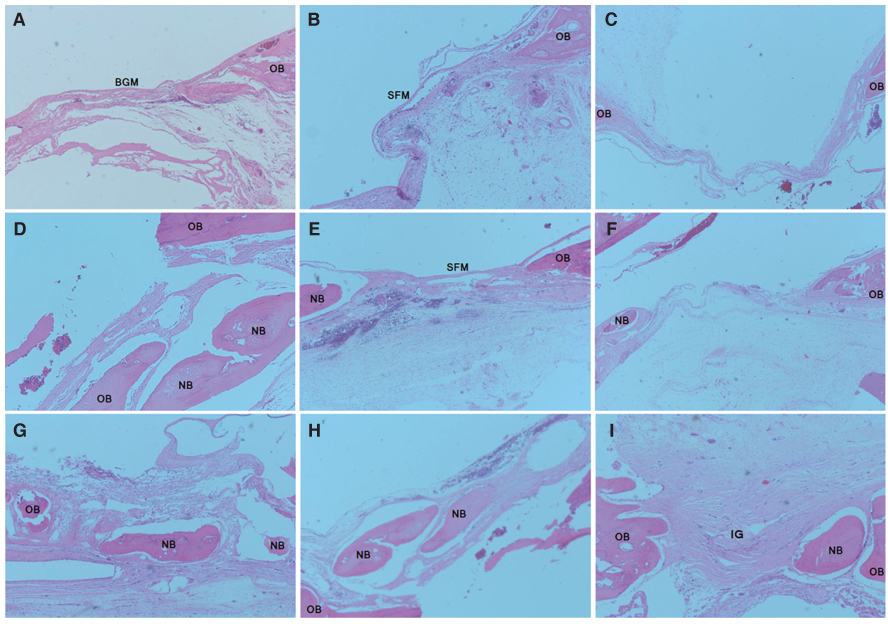
Bone regeneration was observed in the SF and Bio-Gide(R)-treated groups to a greater extent than in the control group (mean volume of new bone was 5.49 +/- 1.48 mm3 at 8 weeks). There were different patterns of bone regeneration between the SF membrane and the Bio-Gide(R) samples. However, the absolute volume of new bone in the SF membrane-treated group was not significantly different from that in the collagen membrane-treated group at 8 weeks (8.75 +/- 0.80 vs. 8.47 +/- 0.75 mm3, respectively, P=.592).
CONCLUSION
SF membranes successfully enhanced comparable volumes of bone regeneration in calvarial bone defects compared with collagen membranes. Considering the lower cost and lesser risk of infectious transmission from animal tissue, SF membranes are a viable alternative to collagen membranes for GBR.
MeSH Terms
Figure
Reference
-
1. Becker BE, Becker W. Regeneration procedures: grafting materials, guided tissue regeneration, and growth factors. Curr Opin Dent. 1991; 1:93–97.2. Karring T, Nyman S, Gottlow J, Laurell L. Development of the biological concept of guided tissue regeneration-animal and human studies. Periodontol 2000. 1993; 1:26–35.3. Hämmerle CH, Jung RE. Bone augmentation by means of barrier membranes. Periodontol 2000. 2003; 33:36–53.4. Dahlin C, Linde A, Gottlow J, Nyman S. Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg. 1988; 81:672–676.5. Linde A, Thorén C, Dahlin C, Sandberg E. Creation of new bone by an osteopromotive membrane technique: an experimental study in rats. J Oral Maxillofac Surg. 1993; 51:892–897.6. Hämmerle CH, Jung RE, Feloutzis A. A systematic review of the survival of implants in bone sites augmented with barrier membranes (guided bone regeneration) in partially edentulous patients. J Clin Periodontol. 2002; 29:226–231.7. Jung RE, Fenner N, Hämmerle CH, Zitzmann NU. Long-term outcome of implants placed with guided bone regeneration (GBR) using resorbable and non-resorbable membranes after 12-14 years. Clin Oral Implants Res. 2013; 24:1065–1073.8. Dahlin C, Sennerby L, Lekholm U, Linde A, Nyman S. Generation of new bone around titanium implants using a membrane technique: an experimental study in rabbits. Int J Oral Maxillofac Implants. 1989; 4:19–25.9. Davarpanah M, Tecucianu JF, Slama M, Celletti R. Bone regeneration in implantology. The use of Gore-Tex membranes: GTAM. J Parodontol. 1991; 10:169–176.10. Machtei EE. The effect of membrane exposure on the outcome of regenerative procedures in humans: a meta-analysis. J Periodontol. 2001; 72:512–516.11. Simion M, Baldoni M, Rossi P, Zaffe D. A comparative study of the effectiveness of e-PTFE membranes with and without early exposure during the healing period. Int J Periodontics Restorative Dent. 1994; 14:166–180.12. Schmidmaier G, Baehr K, Mohr S, Kretschmar M, Beck S, Wildemann B. Biodegradable polylactide membranes for bone defect coverage: biocompatibility testing, radiological and histological evaluation in a sheep model. Clin Oral Implants Res. 2006; 17:439–444.13. Warrer K, Karring T, Nyman S, Gogolewski S. Guided tissue regeneration using biodegradable membranes of polylactic acid or polyurethane. J Clin Periodontol. 1992; 19:633–640.14. Sevor JJ, Meffert RM, Cassingham RJ. Regeneration of dehisced alveolar bone adjacent to endosseous dental implants utilizing a resorbable collagen membrane: clinical and histologic results. Int J Periodontics Restorative Dent. 1993; 13:71–83.15. Tams J, Rozema FR, Bos RR, Roodenburg JL, Nikkels PG, Vermey A. Poly(L-lactide) bone plates and screws for internal fixation of mandibular swing osteotomies. Int J Oral Maxillofac Surg. 1996; 25:20–24.16. Wu L, Ding J. In vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2004; 25:5821–5830.17. Taylor MS, Daniels AU, Andriano KP, Heller J. Six bioabsorbable polymers: in vitro acute toxicity of accumulated degradation products. J Appl Biomater. 1994; 5:151–157.18. Zitzmann NU, Schärer P, Marinello CP. Long-term results of implants treated with guided bone regeneration: a 5-year prospective study. Int J Oral Maxillofac Implants. 2001; 16:355–366.19. Fishman JA, Scobie L, Takeuchi Y. Xenotransplantationassociated infectious risk: a WHO consultation. Xenotransplantation. 2012; 19:72–81.20. Pauli G. Tissue safety in view of CJD and variant CJD. Cell Tissue Bank. 2005; 6:191–200.21. van Leeuwen AC, Huddleston Slater JJ, Gielkens PF, de Jong JR, Grijpma DW, Bos RR. Guided bone regeneration in rat mandibular defects using resorbable poly(trimethylene carbonate) barrier membranes. Acta Biomater. 2012; 8:1422–1429.22. Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-based biomaterials. Biomaterials. 2003; 24:401–416.23. Cao Y, Wang B. Biodegradation of silk biomaterials. Int J Mol Sci. 2009; 10:1514–1524.24. Min BM, Lee G, Kim SH, Nam YS, Lee TS, Park WH. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials. 2004; 25:1289–1297.25. Sofia S, McCarthy MB, Gronowicz G, Kaplan DL. Functionalized silk-based biomaterials for bone formation. J Biomed Mater Res. 2001; 54:139–148.26. Kim KH, Jeong L, Park HN, Shin SY, Park WH, Lee SC, Kim TI, Park YJ, Seol YJ, Lee YM, Ku Y, Rhyu IC, Han SB, Chung CP. Biological efficacy of silk fibroin nanofiber membranes for guided bone regeneration. J Biotechnol. 2005; 120:327–339.27. Bosch C, Melsen B, Vargervik K. Importance of the criticalsize bone defect in testing bone-regenerating materials. J Craniofac Surg. 1998; 9:310–316.28. Gielkens PF, Schortinghuis J, de Jong JR, Raghoebar GM, Stegenga B, Bos RR. Vivosorb, Bio-Gide, and Gore-Tex as barrier membranes in rat mandibular defects: an evaluation by microradiography and micro-CT. Clin Oral Implants Res. 2008; 19:516–521.29. Rüegsegger P, Koller B, Müller R. A microtomographic system for the nondestructive evaluation of bone architecture. Calcif Tissue Int. 1996; 58:24–29.30. Dalstra M, Verna C, Cacciafesta V, Andreassen TT, Melsen B. Micro-computed tomography to evaluate bone remodeling and mineralization. Adv Exp Med Biol. 2001; 496:9–19.31. Verna C, Dalstra M, Wikesjö UM, Trombelli L. Carles Bosch. Healing patterns in calvarial bone defects following guided bone regeneration in rats. A micro-CT scan analysis. J Clin Periodontol. 2002; 29:865–870.32. Coelho PG, Giro G, Kim W, Granato R, Marin C, Bonfante EA, Bonfante S, Lilin T, Suzuki M. Evaluation of collagenbased membranes for guided bone regeneration, by three-dimensional computerized microtomography. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012; 114:437–443.33. Gielkens PF, Schortinghuis J, de Jong JR, Huysmans MC, Leeuwen MB, Raghoebar GM, Bos RR, Stegenga B. A comparison of micro-CT, microradiography and histomorphometry in bone research. Arch Oral Biol. 2008; 53:558–566.34. Dahlin C, Andersson L, Linde A. Bone augmentation at fenestrated implants by an osteopromotive membrane technique. A controlled clinical study. Clin Oral Implants Res. 1991; 2:159–165.35. Uebersax L, Hagenmüller H, Hofmann S, Gruenblatt E, Müller R, Vunjak-Novakovic G, Kaplan DL, Merkle HP, Meinel L. Effect of scaffold design on bone morphology in vitro. Tissue Eng. 2006; 12:3417–3429.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Tetracycline-loaded Silk Fibroin Membrane on Guided Bone Regeneration in a Rabbit Calvarial Defect Model
- The Effect of Silk Fibroin Particles Coated with Hydroxyapatites on Bone Regeneration in the Rat Calvarial Defect Model
- Membranes for the Guided Bone Regeneration
- Factors Influencing Regeneration of Calvarial Defects in Rats
- A comparative study for guided bone regeneration of silk fibroin nanomembrane(NanoGide-S(TM))