Clin Exp Otorhinolaryngol.
2014 Mar;7(1):47-52.
Favorable Vocal Fold Wound Healing Induced by Platelet-Rich Plasma Injection
- Affiliations
-
- 1Department of Otolaryngology, Gyeongsang National University School of Medicine, Jinju, Korea.
- 2Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju, Korea.
- 3Department of Otorhinolaryngology-Head and Neck Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Department of Laboratory Medicine, Gyeongsang National University School of Medicine, Jinju, Korea.
- 5Department of Otorhinolaryngology-Head and Neck Surgery, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. lesaby@daum.com
- 6Department of Pathology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
Abstract
OBJECTIVES
To introduce a new injection material for vocal fold diseases, which could be readily translated to clinical practice, we investigated the effectiveness of platelet-rich plasma (PRP) injection on the injured vocal fold in terms of histological recovery.
METHODS
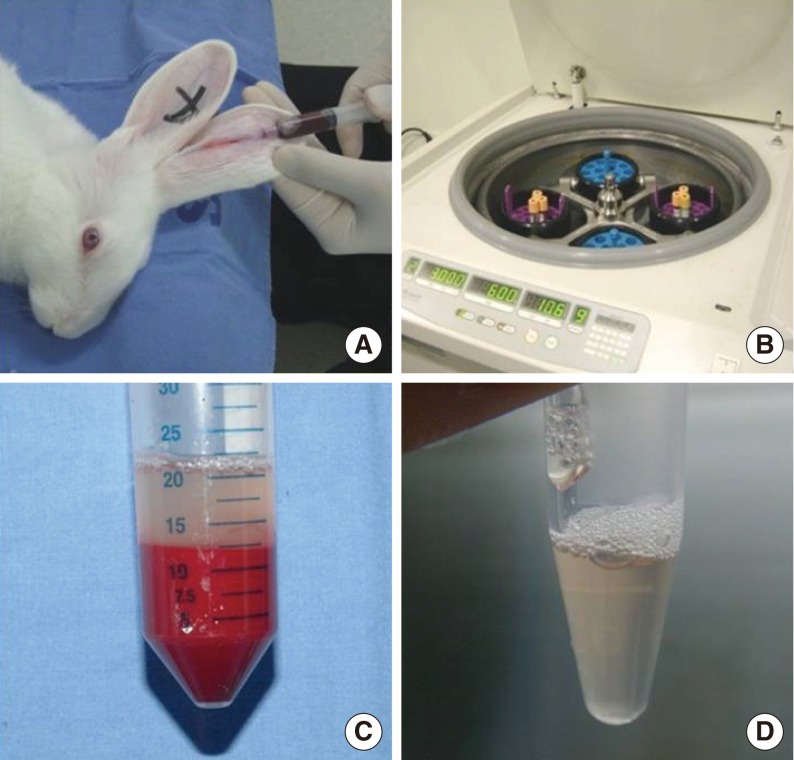
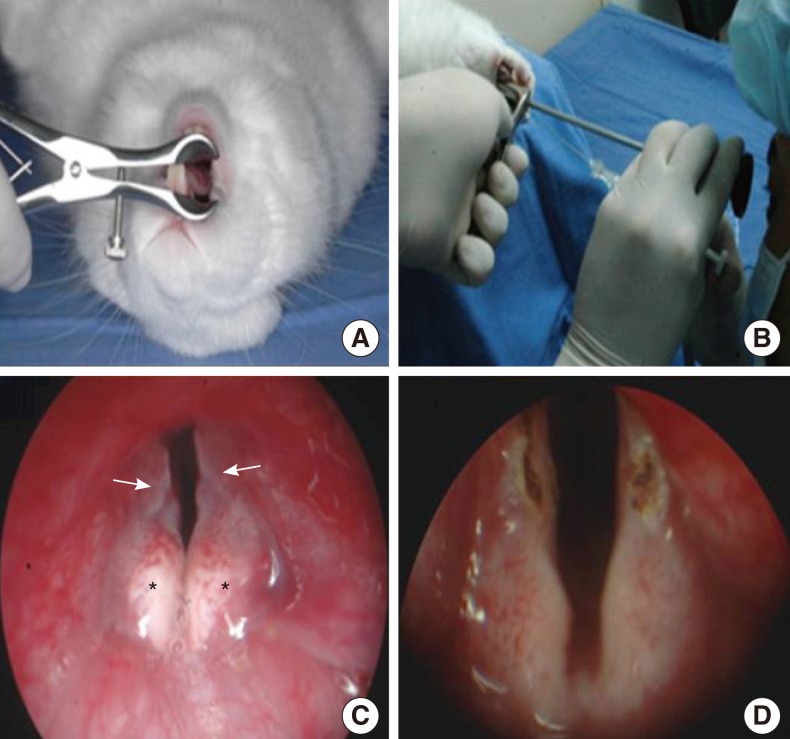
Blood samples were drawn from New Zealand White rabbits and PRP was isolated through centrifugation and separation of the samples. Using a CO2 laser, we made a linear wound in the 24 vocal fold sides of 12 rabbits and injected each wound with PRP on one vocal fold side and normal saline (NS) on the other. Morphologic analyses were conducted at 2, 4, and 12 weeks after injection, and inflammatory response, collagen deposit, and changes in growth factors were assessed using H&E and masson trichrome (MT) staining and western blot assay.
RESULTS
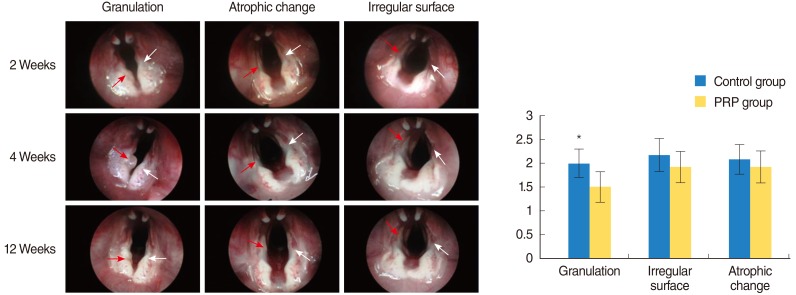
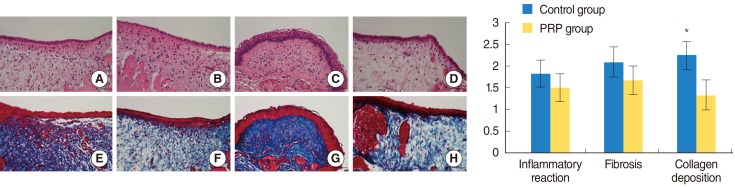
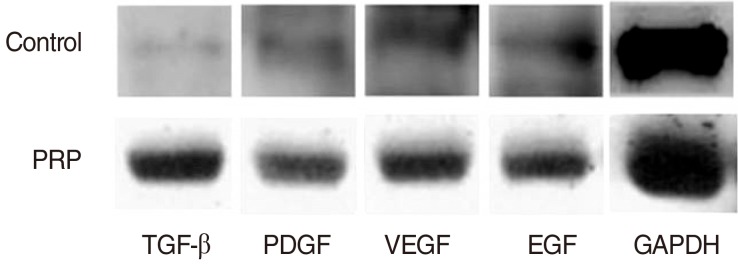
PRP was prepared in approximately 40 minutes. The mean platelet concentration was 1,315,000 platelets/mm3. In morphological analyses, decreased granulation was observed in the PRP-injected vocal folds (P<0.05). However, the irregular surface and atrophic change were not difference. Histological findings revealed significant inflammation and collagen deposition in NS-injected vocal folds, whereas the PRP-injected vocal folds exhibited less (P<0.05). However, the inflammatory reaction and fibrosis were not difference. In western blot assay, increased amounts of growth factors were observed in PRP-injected vocal folds.
CONCLUSION
Injection of injured rabbit vocal folds with PRP led to improved wound healing and fewer signs of scarring as demonstrated by decreased inflammation and collagen deposition. The increased vocal fold regeneration may be due to the growth factors associated with PRP.
Keyword
MeSH Terms
Figure
Reference
-
1. Bless DM, Welham NV. Characterization of vocal fold scar formation, prophylaxis, and treatment using animal models. Curr Opin Otolaryngol Head Neck Surg. 2010; 12. 18(6):481–486. PMID: 20962643.
Article2. Woodal J Jr, Tucci M, Mishra A, Benghuzzi H. Cellular effects of platelet rich plasma: a study on HL-60 macrophage-like cells. Biomed Sci Instrum. 2007; 43:266–271. PMID: 17487092.3. Yol S, Tekin A, Yilmaz H, Kucukkartallar T, Esen H, Caglayan O, et al. Effects of platelet rich plasma on colonic anastomosis. J Surg Res. 2008; 5. 146(2):190–194. PMID: 18028949.
Article4. Behnke O, Forer A. From megakaryocytes to platelets: platelet morphogenesis takes place in the bloodstream. Eur J Haematol Suppl. 1998; 61:3–23. PMID: 9658684.
Article5. Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004; 1. 9:283–289. PMID: 14766366.
Article6. Brecher G, Cronkite EP. Morphology and enumeration of human blood platelets. J Appl Physiol. 1950; 12. 3(6):365–377. PMID: 14794596.
Article7. Brecher G, Schneiderman M, Cronkite EP. The reproducibility and constancy of the platelet count. Am J Clin Pathol. 1953; 1. 23(1):15–26. PMID: 13016502.
Article8. Branski RC, Rosen CA, Verdolini K, Hebda PA. Acute vocal fold wound healing in a rabbit model. Ann Otol Rhinol Laryngol. 2005; 1. 114(1 Pt 1):19–24. PMID: 15697158.
Article9. Branski RC, Rosen CA, Verdolini K, Hebda PA. Biochemical markers associated with acute vocal fold wound healing: a rabbit model. J Voice. 2005; 6. 19(2):283–289. PMID: 15907442.
Article10. Peled ZM, Chin GS, Liu W, Galliano R, Longaker MT. Response to tissue injury. Clin Plast Surg. 2000; 10. 27(4):489–500. PMID: 11039884.
Article11. Mallur PS, Rosen CA. Vocal fold injection: review of indications, techniques, and materials for augmentation. Clin Exp Otorhinolaryngol. 2010; 12. 3(4):177–182. PMID: 21217957.
Article12. Correa AJ, Reinisch L, Sanders DL, Huang S, Deriso W, Duncavage JA, et al. Inhibition of subglottic stenosis with mitomycin-C in the canine model. Ann Otol Rhinol Laryngol. 1999; 11. 108(11 Pt 1):1053–1060. PMID: 10579232.
Article13. Eliashar R, Eliachar I, Esclamado R, Gramlich T, Strome M. Can topical mitomycin prevent laryngotracheal stenosis? Laryngoscope. 1999; 10. 109(10):1594–1600. PMID: 10522927.
Article14. Garrett CG, Soto J, Riddick J, Billante CR, Reinisch L. Effect of mitomycin-C on vocal fold healing in a canine model. Ann Otol Rhinol Laryngol. 2001; 1. 110(1):25–30. PMID: 11201804.
Article15. Gray SD, Tritle N, Li W. The effect of mitomycin on extracellular matrix proteins in a rat wound model. Laryngoscope. 2003; 2. 113(2):237–242. PMID: 12567075.
Article16. Matsumoto K, Nakamura T. Hepatocyte growth factor (HGF) as a tissue organizer for organogenesis and regeneration. Biochem Biophys Res Commun. 1997; 10. 239(3):639–644. PMID: 9367820.
Article17. Hirano S, Kishimoto Y, Suehiro A, Kanemaru S, Ito J. Regeneration of aged vocal fold: first human case treated with fibroblast growth factor. Laryngoscope. 2008; 12. 118(12):2254–2259. PMID: 19029860.
Article18. Hirano S, Bless D, Heisey D, Ford C. Roles of hepatocyte growth factor and transforming growth factor beta1 in production of extracellular matrix by canine vocal fold fibroblasts. Laryngoscope. 2003; 1. 113(1):144–148. PMID: 12514399.19. Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004; 11. 114(6):1502–1508. PMID: 15509939.
Article20. Atri SC, Misra J, Bisht D, Misra K. Use of homologous platelet factors in achieving total healing of recalcitrant skin ulcers. Surgery. 1990; 9. 108(3):508–512. PMID: 2396195.21. Doucette MM, Fylling C, Knighton DR. Amputation prevention in a high-risk population through comprehensive wound-healing protocol. Arch Phys Med Rehabil. 1989; 10. 70(10):780–785. PMID: 2802960.22. Glover JL, Weingarten MS, Buchbinder DS, Poucher RL, Deitrick GA 3rd, Fylling CP. A 4-year outcome-based retrospective study of wound healing and limb salvage in patients with chronic wounds. Adv Wound Care. 1997; Jan-Feb. 10(1):33–38. PMID: 9204802.23. Keyser JE. Diabetic wound healing and limb salvage in an outpatient wound care program. South Med J. 1993; 3. 86(3):311–317. PMID: 8451671.
Article24. Grageda E. Platelet-rich plasma and bone graft materials: a review and a standardized research protocol. Implant Dent. 2004; 12. 13(4):301–309. PMID: 15591991.
Article25. Kassolis JD, Reynolds MA. Evaluation of the adjunctive benefits of platelet-rich plasma in subantral sinus augmentation. J Craniofac Surg. 2005; 3. 16(2):280–287. PMID: 15750426.
Article26. Weibrich G, Hansen T, Kleis W, Buch R, Hitzler WE. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone. 2004; 4. 34(4):665–671. PMID: 15050897.
Article27. Woo SH, Son YI, Lee SH, Park JJ, Kim JP. Comparative analysis on the efficiency of the injection laryngoplasty technique using calcium hydroxyapatite (CaHA): the thyrohyoid approach versus the cricothyroid approach. J Voice. 2013; 3. 27(2):236–241. PMID: 23280385.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Platelet-rich Plasma on Burn Wounds according to Time of Application: An Experimental Study on Rats
- Vocal Fold Injection: Review of Indications, Techniques, and Materials for Augmentation
- Acceleration of Wound Healing Using Adipose-derived Stem Cell Therapy with Platelet Concentrates: Platelet-rich Plasma (PRP) vs. Platelet-rich Fibrin (PRF)
- Successful Treatment of Nicolau Syndrome with Platelet Rich Plasma
- Intralesional Injection of Autologous Platelet-Rich Plasma as an Effective Regeneration Therapy: A Case Report of Chronic Wagner Grade 2 Diabetic Foot Ulcer