Korean J Obstet Gynecol.
2011 Sep;54(9):499-507. 10.5468/KJOG.2011.54.9.499.
The effect of estrogen on extracellular signal-regulated kinases activation in uterine artery smooth muscle cells during pregnancy
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Hallym University College of Medicine, Seoul, Korea. obgyn25@hallym.or.kr
- KMID: 2274074
- DOI: http://doi.org/10.5468/KJOG.2011.54.9.499
Abstract
OBJECTIVE
To explore the endothelium-independent mechanisms of estrogen induced uterine vasodilatation, this study was performed to determine whether uterine artery smooth muscle (UASM) cells are direct targets of estrogen, and estradiol (E2) stimulates extracellular signal-regulated kinases (ERK2/1) in endothelium denuded UASMs.
METHODS
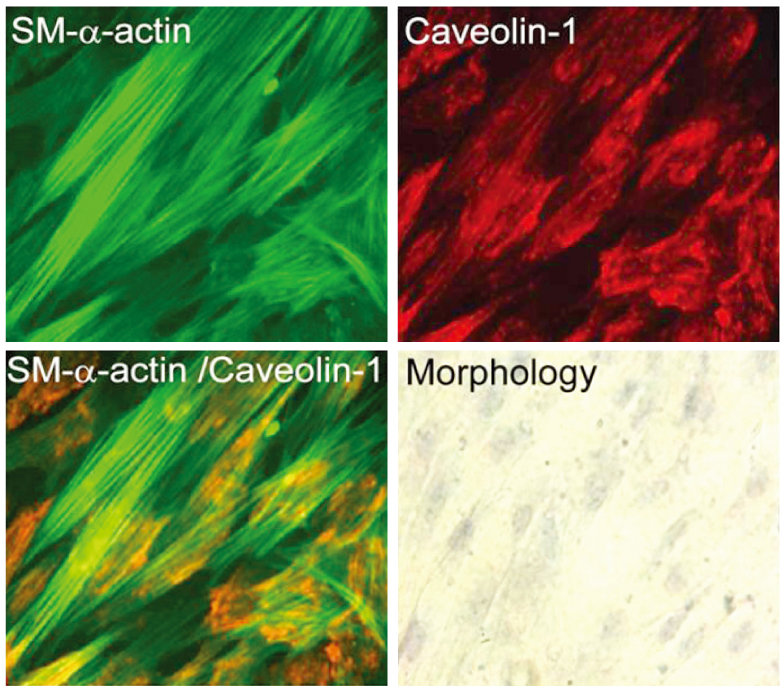
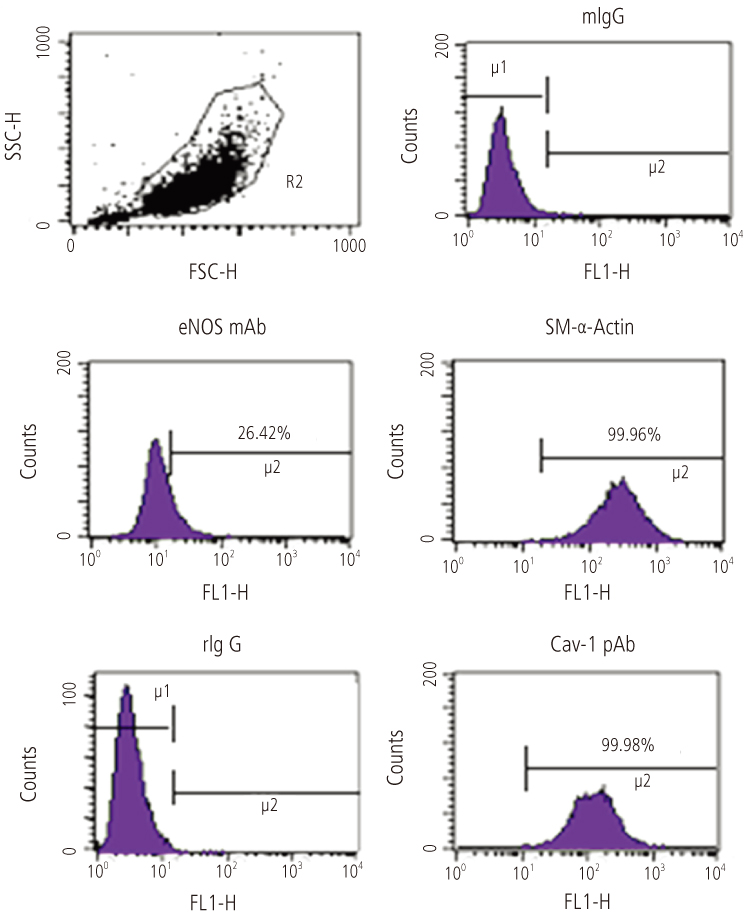
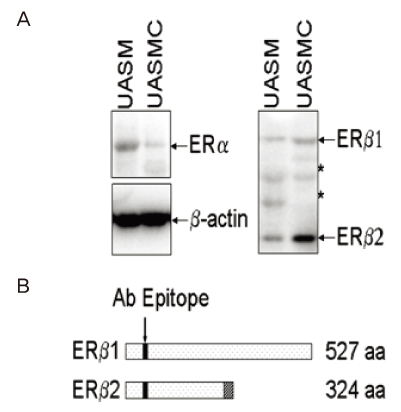
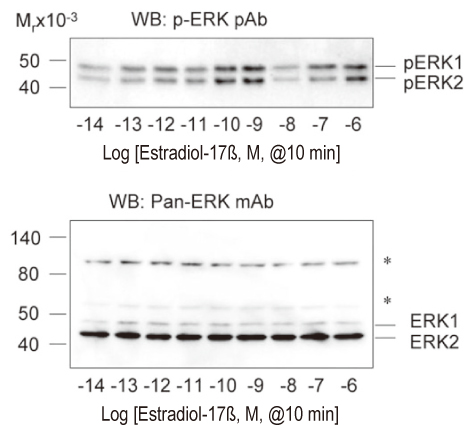
The uterine arteries were obtained from late gestation pregnant sheep and the endothelium was denuded with collagenase digestion. The uterine artery smooth muscle segments were digested, collected and cultured. Endothelial integrity and smooth muscle status were assessed by Double immunofluorescence staining and flow cytometry. The UASM cells were treated with increasing concentrations of E2 (10(-14) to 10(-6) M), and pretreated with ICI 182,780 followed by different concentrations (10(-10) and 10(-7) M) of E2. Western blot analysis of ERK2/1 phosphorylation with a phospho-mitogen activated protein kinases (MAPKs) antibody were carried out to total cell extracts.
RESULTS
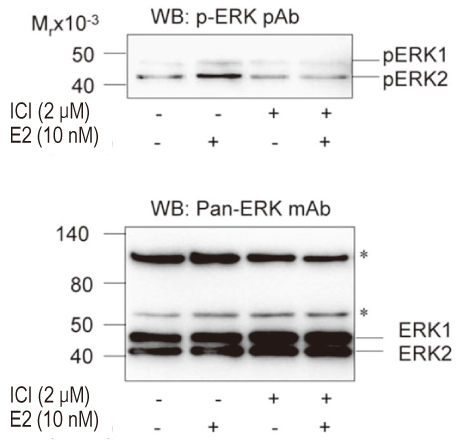
The loss of endothelial function and adequacy of smooth muscle integrity were confirmed. When challenged with increasing concentrations of E2, a bi-phase ERK2/1 phosphorylation was observed. Treatment with low doses (10(-14) to 10(-10) M) of E2, ERK2/1 phosphorylation was dose-dependently increased, whereas high doses (10(-9) to 10(-8) M) did not phosphorylate ERK2/1. However, treatment with pharmacological doses (10(-7) to 10(-6) M) drastically phosphorylated ERK2/1. In the presence of ICI 182,780, E2 induced ERK2/1 phosphorylation were abolished in both.
CONCLUSION
It suggests that UASM is the target tissue of estrogen during uterine vasodilatation, and estrogen stimulation of ERK2/1 activation is mediated by an estrogen receptor-dependent mechanism. It also is presumed that endothelium independent mechanism exists in estrogen induced vasodilatation.
Keyword
MeSH Terms
-
Blotting, Western
Collagenases
Digestion
Endothelium
Estradiol
Estrogens
Extracellular Signal-Regulated MAP Kinases
Flow Cytometry
Fluorescent Antibody Technique
Muscle, Smooth
Myocytes, Smooth Muscle
Phosphorylation
Pregnancy
Protein Kinases
Sheep
Uterine Artery
Vasodilation
Collagenases
Estradiol
Estrogens
Extracellular Signal-Regulated MAP Kinases
Protein Kinases
Figure
Reference
-
1. Robson SC, Hunter S, Boys RJ, Dunlop W. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol. 1989. 256:H1060–H1065.2. Clapp JF 3rd, Capeless E. Cardiovascular function before, during, and after the first and subsequent pregnancies. Am J Cardiol. 1997. 80:1469–1473.3. Slangen BF, Out IC, Verkeste CM, Peeters LL. Hemodynamic changes in early pregnancy in chronically instrumented, conscious rats. Am J Physiol. 1996. 270:H1779–H1784.4. Chapman AB, Abraham WT, Zamudio S, Coffin C, Merouani A, Young D, et al. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int. 1998. 54:2056–2063.5. van Oppen AC, van der Tweel I, Alsbach GP, Heethaar RM, Bruinse HW. A longitudinal study of maternal hemodynamics during normal pregnancy. Obstet Gynecol. 1996. 88:40–46.6. Wong AY, Kulandavelu S, Whiteley KJ, Qu D, Langille BL, Adamson SL. Maternal cardiovascular changes during pregnancy and postpartum in mice. Am J Physiol Heart Circ Physiol. 2002. 282:H918–H925.7. Duvekot JJ, Peeters LL. Renal hemodynamics and volume homeostasis in pregnancy. Obstet Gynecol Surv. 1994. 49:830–839.8. Duvekot JJ, Cheriex EC, Pieters FA, Menheere PP, Peeters LH. Early pregnancy changes in hemodynamics and volume homeostasis are consecutive adjustments triggered by a primary fall in systemic vascular tone. Am J Obstet Gynecol. 1993. 169:1382–1392.9. Spaanderman ME, Meertens M, van Bussel M, Ekhart TH, Peeters LL. Cardiac output increases independently of basal metabolic rate in early human pregnancy. Am J Physiol Heart Circ Physiol. 2000. 278:H1585–H1588.10. Magness RR, Zheng J. Gluckman PD, Heymann MA, editors. Maternal cardiovascular alteration during pregnancy. Pediatric and perinatal perspectives: the scientific basis. 1996. London: 762–772.11. Rosenfeld CR. Rosenfeld CR, editor. Changes in uterine blood flow during pregnancy. Reproductive and perinatal medicine: the uterine circulation. 1989. vol. X. Ithaca (NY): Perinatology Press;135–156.12. Magness RR, Rosenfeld CR. Rosenfeld CR, editor. The role of steroid hormones in the control of uterine blood flow. Reproductive and perinatal medicine: the uterine circulation. 1989. vol. X. Ithaca (NY): Perinatology Press;239–271.13. Ford SP. Control of uterine and ovarian blood flow throughout the estrous cycle and pregnancy of ewes, sows and cows. J Anim Sci. 1982. 55:Suppl 2. 32–42.14. Van Buren GA, Yang DS, Clark KE. Estrogen-induced uterine vasodilatation is antagonized by L-nitroarginine methyl ester, an inhibitor of nitric oxide synthesis. Am J Obstet Gynecol. 1992. 167:828–833.15. Rosenfeld CR, Cox BE, Roy T, Magness RR. Nitric oxide contributes to estrogen-induced vasodilation of the ovine uterine circulation. J Clin Invest. 1996. 98:2158–2166.16. Magness RR, Sullivan JA, Li Y, Phernetton TM, Bird IM. Endothelial vasodilator production by uterine and systemic arteries. VI. Ovarian and pregnancy effects on eNOS and NO(x). Am J Physiol Heart Circ Physiol. 2001. 280:H1692–H1698.17. Rupnow HL, Phernetton TM, Shaw CE, Modrick ML, Bird IM, Magness RR. Endothelial vasodilator production by uterine and systemic arteries. VII. Estrogen and progesterone effects on eNOS. Am J Physiol Heart Circ Physiol. 2001. 280:H1699–H1705.18. Chen DB, Bird IM, Zheng J, Magness RR. Membrane estrogen receptor-dependent extracellular signal-regulated kinase pathway mediates acute activation of endothelial nitric oxide synthase by estrogen in uterine artery endothelial cells. Endocrinology. 2004. 145:113–125.19. Moon ZS, Park HM, Hur M, Lee MY. The effect of progestogens on the tone of human vascular smooth muscles. Korean J Obstet Gynecol. 2001. 44:714–726.20. Chester AH, Jiang C, Borland JA, Yacoub MH, Collins P. Oestrogen relaxes human epicardial coronary arteries through non-endothelium-dependent mechanisms. Coron Artery Dis. 1995. 6:417–422.21. Bird IM, Sullivan JA, Di T, Cale JM, Zhang L, Zheng J, et al. Pregnancy-dependent changes in cell signaling underlie changes in differential control of vasodilator production in uterine artery endothelial cells. Endocrinology. 2000. 141:1107–1117.22. Cho A, Courtman DW, Langille BL. Apoptosis (programmed cell death) in arteries of the neonatal lamb. Circ Res. 1995. 76:168–175.23. Wong LC, Langille BL. Developmental remodeling of the internal elastic lamina of rabbit arteries: effect of blood flow. Circ Res. 1996. 78:799–805.24. Dzau VJ, Gibbons GH. Vascular remodeling: mechanisms and implications. J Cardiovasc Pharmacol. 1993. 21:Suppl 1. S1–S5.25. Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994. 330:1431–1438.26. Langille BL. Arterial remodeling: relation to hemodynamics. Can J Physiol Pharmacol. 1996. 74:834–841.27. Salhab WA, Shaul PW, Cox BE, Rosenfeld CR. Regulation of types I and III NOS in ovine uterine arteries by daily and acute estrogen exposure. Am J Physiol Heart Circ Physiol. 2000. 278:H2134–H2142.28. Langille BL, O'Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science. 1986. 231:405–407.29. Michel T, Feron O. Nitric oxide synthases: which, where, how, and why? J Clin Invest. 1997. 100:2146–2152.30. Albrecht EW, Stegeman CA, Heeringa P, Henning RH, van Goor H. Protective role of endothelial nitric oxide synthase. J Pathol. 2003. 199:8–17.31. MacRitchie AN, Jun SS, Chen Z, German Z, Yuhanna IS, Sherman TS, et al. Estrogen upregulates endothelial nitric oxide synthase gene expression in fetal pulmonary artery endothelium. Circ Res. 1997. 81:355–362.32. Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol. 1986. 250:H1145–H1149.33. Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension. 1986. 8:37–44.34. Chen D, Zangl AL, Zhao Q, Markley JL, Zheng J, Bird IM, et al. Ovine caveolin-1: cDNA cloning, E. coli expression, and association with endothelial nitric oxide synthase. Mol Cell Endocrinol. 2001. 175:41–56.35. Peterson TE, Guicciardi ME, Gulati R, Kleppe LS, Mueske CS, Mookadam M, et al. Caveolin-1 can regulate vascular smooth muscle cell fate by switching platelet-derived growth factor signaling from a proliferative to an apoptotic pathway. Arterioscler Thromb Vasc Biol. 2003. 23:1521–1527.36. Ihionkhan CE, Chambliss KL, Gibson LL, Hahner LD, Mendelsohn ME, Shaul PW. Estrogen causes dynamic alterations in endothelial estrogen receptor expression. Circ Res. 2002. 91:814–820.37. Liao WX, Magness RR, Chen DB. Expression of estrogen receptors-alpha and -beta in the pregnant ovine uterine artery endothelial cells in vivo and in vitro. Biol Reprod. 2005. 72:530–537.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Phytoestrogen-Induced Phosphorylation of MAP Kinase in Osteoblasts is Mediated by Membrane Estrogen Receptor
- Sustained Intracellular Acidosis Triggers the Naâº/H⺠Exchager-1 Activation in Glutamate Excitotoxicity
- Lobaric Acid Inhibits VCAM-1 Expression in TNF-alpha-Stimulated Vascular Smooth Muscle Cells via Modulation of NF-kappaB and MAPK Signaling Pathways
- Proliferative and Synthetic Responses of Airway Smooth Muscle in Asthma
- Effect of Mild Hypothermia on the Mitogen Activated Protein Kinases in Experimental Stroke