Allergy Asthma Immunol Res.
2015 May;7(3):276-282. 10.4168/aair.2015.7.3.276.
Inhibitory Effect of Delphinidin on Extracellular Matrix Production via the MAPK/NF-kappaB Pathway in Nasal Polyp-Derived Fibroblasts
- Affiliations
-
- 1Brain Korea 21 Plus for Biomedical Science, Korea University, College of Medicine, Seoul, Korea. lhman@korea.ac.kr
- 2Institute for Medical Devices Clinical Trial Center, Korea University, College of Medicine, Seoul, Korea.
- 3Guro Hospital Department of Otorhinolaryngology Head and Neck Surgery, Korea University, College of Medicine, Seoul, Korea.
- KMID: 2260465
- DOI: http://doi.org/10.4168/aair.2015.7.3.276
Abstract
- PURPOSE
Nasal polyps are associated with chronic inflammation of the mucous membranes in the nose and paranasal sinuses and involved in extracellular matrix (ECM) accumulation. Delphinidin promotes ECM degradation in hepatitis and cardiac fibrosis. The aims of this study were to examine the inhibitory effect of delphinidin on TGF-beta1-induced myofibroblast differentiation and ECM accumulation, and to determine the underlying mechanisms in nasal polyp-derived fibroblasts (NPDFs).
METHODS
NPDFs were stimulated with TGF-beta1, with or without delphinidin, and the expression levels of alpha-SMA, fibronectin, and collagen type I were determined by RT-PCR, Western blot analysis, and collagen assay. The expression of alpha-SMA protein was measured by immunocytochemical staining. Mitogen-activated protein kinase and NF-kappaB activation induced by TGF-beta1 were determined by Western blot analysis. The transcriptional activity of NF-kappaB was measured by luciferase assay.
RESULTS
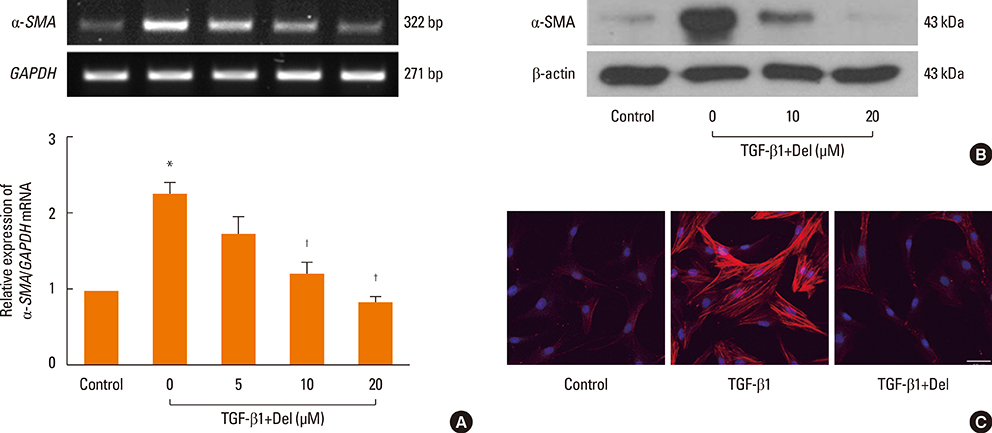
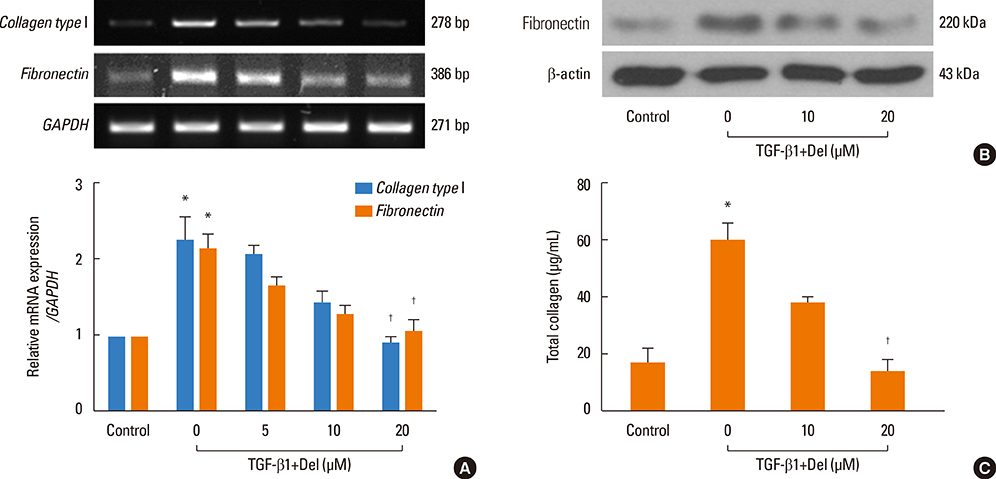
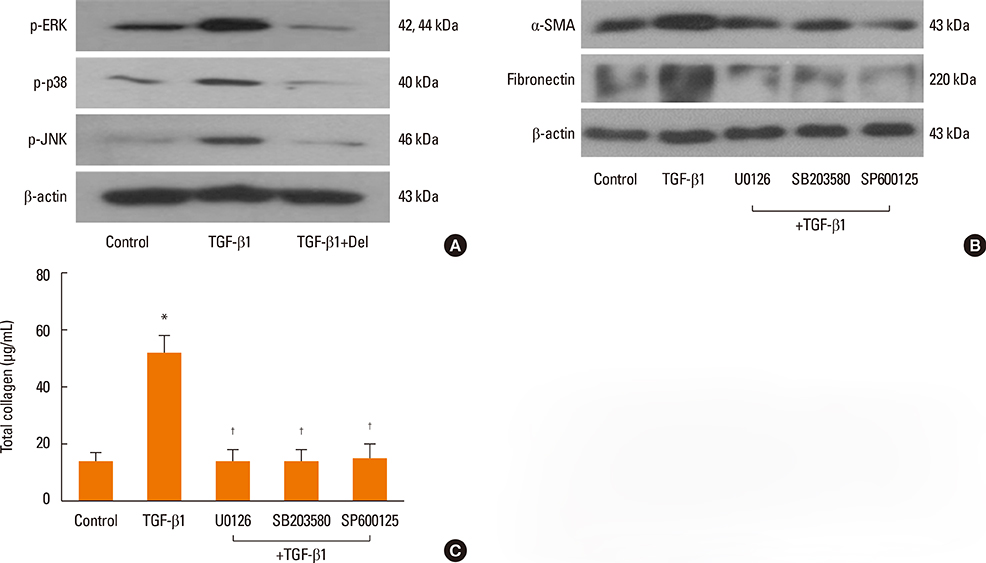
The expression levels of alpha-SMA, fibronectin, and collagen type I increased in TGF-beta1-stimulated NPDFs. In TGF-beta1-induced NPDFs, delphinidin inhibited the expression of alpha-SMA, fibronectin, and collagen. Inhibitors of MAPK and NF-kappaB blocked the expression of alpha-SMA, fibronectin, and collagen type I. Delphinidin suppressed the activation of MAPK and NF-kappaB induced by TGF-beta1 stimulation.
CONCLUSIONS
These results suggest that delphinidin may inhibit TGF-beta1-induced myofibroblast differentiation and ECM production through the MAPK/NF-kappaB signaling pathway in NPDFs.
Keyword
MeSH Terms
-
Blotting, Western
Collagen
Collagen Type I
Extracellular Matrix*
Fibroblasts*
Fibronectins
Fibrosis
Hepatitis
Inflammation
Luciferases
Mucous Membrane
Myofibroblasts
Nasal Polyps
NF-kappa B
Nose
Paranasal Sinuses
Protein Kinases
Transforming Growth Factor beta1
Collagen
Collagen Type I
Fibronectins
Luciferases
NF-kappa B
Protein Kinases
Transforming Growth Factor beta1
Figure
Reference
-
1. Pawankar R. Nasal polyposis: an update: editorial review. Curr Opin Allergy Clin Immunol. 2003; 3:1–6.2. Chaaban MR, Walsh EM, Woodworth BA. Epidemiology and differential diagnosis of nasal polyps. Am J Rhinol Allergy. 2013; 27:473–478.3. Rinia AB, Kostamo K, Ebbens FA, van Drunen CM, Fokkens WJ. Nasal polyposis: a cellular-based approach to answering questions. Allergy. 2007; 62:348–358.4. Kendall RT, Feghali-Bostwick CA. Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol. 2014; 5:123.5. Haruna S, Nakanishi M, Otori N, Moriyama H. Histopathological features of nasal polyps with asthma association: an immunohistochemical study. Am J Rhinol. 2004; 18:165–172.6. Serpero L, Petecchia L, Sabatini F, Giuliani M, Silvestri M, Di Blasi P, et al. The effect of transforming growth factor (TGF)-beta1 and (TGF)-beta2 on nasal polyp fibroblast activities involved upper airway remodeling: modulation by fluticasone propionate. Immunol Lett. 2006; 105:61–67.7. Little SC, Early SB, Woodard CR, Shonka DC Jr, Han JK, Borish L, et al. Dual action of TGF-beta1 on nasal-polyp derived fibroblasts. Laryngoscope. 2008; 118:320–324.8. Van Bruaene N, Derycke L, Perez-Novo CA, Gevaert P, Holtappels G, De Ruyck N, et al. TGF-beta signaling and collagen deposition in chronic rhinosinusitis. J Allergy Clin Immunol. 2009; 124:253–259. 259.e1–259.e2.9. Park HH, Park IH, Cho JS, Lee YM, Lee HM. The effect of macrolides on myofibroblast differentiation and collagen production in nasal polyp-derived fibroblasts. Am J Rhinol Allergy. 2010; 24:348–353.10. Afaq F, Syed DN, Malik A, Hadi N, Sarfaraz S, Kweon MH, et al. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCaT keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis. J Invest Dermatol. 2007; 127:222–232.11. Seong AR, Yoo JY, Choi K, Lee MH, Lee YH, Lee J, et al. Delphinidin, a specific inhibitor of histone acetyltransferase, suppresses inflammatory signaling via prevention of NF-κB acetylation in fibroblast-like synoviocyte MH7A cells. Biochem Biophys Res Commun. 2011; 410:581–586.12. Domitrović R, Jakovac H. Antifibrotic activity of anthocyanidin delphinidin in carbon tetrachloride-induced hepatotoxicity in mice. Toxicology. 2010; 272:1–10.13. Meng J, Zhou P, Liu Y, Liu F, Yi X, Liu S, et al. The development of nasal polyp disease involves early nasal mucosal inflammation and remodelling. PLoS One. 2013; 8:e82373.14. Watelet JB, Claeys C, Perez-Novo C, Gevaert P, Van Cauwenberge P, Bachert C. Transforming growth factor beta1 in nasal remodeling: differences between chronic rhinosinusitis and nasal polyposis. Am J Rhinol. 2004; 18:267–272.15. Czaja AJ. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World J Gastroenterol. 2014; 20:2515–2532.16. Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 2002; 96:67–202.17. Noda Y, Kaneyuki T, Mori A, Packer L. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: delphinidin, cyanidin, and pelargonidin. J Agric Food Chem. 2002; 50:166–171.18. Cho JS, Moon YM, Park IH, Um JY, Moon JH, Park SJ, et al. Epigenetic regulation of myofibroblast differentiation and extracellular matrix production in nasal polyp-derived fibroblasts. Clin Exp Allergy. 2012; 42:872–882.19. Cho JS, Moon YM, Um JY, Moon JH, Park IH, Lee HM. Inhibitory effect of ginsenoside Rg1 on extracellular matrix production via extracellular signal-regulated protein kinase/activator protein 1 pathway in nasal polyp-derived fibroblasts. Exp Biol Med (Maywood). 2012; 237:663–669.20. Harris WT, Kelly DR, Zhou Y, Wang D, MacEwen M, Hagood JS, et al. Myofibroblast differentiation and enhanced TGF-B signaling in cystic fibrosis lung disease. PLoS One. 2013; 8:e70196.21. Kolb M, Bonniaud P, Galt T, Sime PJ, Kelly MM, Margetts PJ, et al. Differences in the fibrogenic response after transfer of active transforming growth factor-beta1 gene to lungs of "fibrosis-prone" and "fibrosis-resistant" mouse strains. Am J Respir Cell Mol Biol. 2002; 27:141–150.22. Barbaro V, Ferrari S, Fasolo A, Ponzin D, Di Iorio E. Reconstruction of a human hemicornea through natural scaffolds compatible with the growth of corneal epithelial stem cells and stromal keratocytes. Mol Vis. 2009; 15:2084–2093.23. Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000; 342:1350–1358.24. Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005; 118:3573–3584.25. Samarakoon R, Overstreet JM, Higgins PJ. TGF-β signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cell Signal. 2013; 25:264–268.26. Kubiczkova L, Sedlarikova L, Hajek R, Sevcikova S. TGF-β-an excellent servant but a bad master. J Transl Med. 2012; 10:183.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Manuka Honey on Transforming Growth Factor Beta-1-Induced Extracelluar Matrix Production in Nasal Polyp Derived Fibroblasts
- Prostaglandin E2 Induces IL-6 and IL-8 Production by the EP Receptors/Akt/NF-kappaB Pathways in Nasal Polyp-Derived Fibroblasts
- Epigenetic Regulation of Nasal Polyp Formation
- Cooperative effect of Alternaria and rhinovirus on the activation of nasal polyp epithelial cells
- p38 MAPK and NF-kappaB are required for LPS-induced RANTES production in immortalized murine microglia (BV-2)