Allergy Asthma Immunol Res.
2013 Nov;5(6):409-414. 10.4168/aair.2013.5.6.409.
The Influence of IgE on Cultured Human Mast Cells
- Affiliations
-
- 1Pediatric Research Lab, Aarhus University Hospital, Denmark. munk_frandsen@hotmail.com
- 2Clinical Institute & Department of Respiratory Medicine, Aarhus University Hospital, Denmark.
- KMID: 2260286
- DOI: http://doi.org/10.4168/aair.2013.5.6.409
Abstract
- PURPOSE
The mast cell plays a pivotal role in the human immune response. Crosslinking of 2 IgE molecules bound to the high affinity IgE receptor (FcepsilonRI) on the surface of the mast cell results in mast cell degranulation and the release of several proinflammatory mediators. Patients with type-I allergy have increased levels of IgE in the blood compared to healthy individuals.
METHODS
In a 6-week culture system of stem cells to human mast cells we investigated the effect of the concentration of IgE. The mast cells were cultured with different concentrations of IgE for the last 10 days of the maturation period. It was observed how the IgE concentration affects the histamine release, FcepsilonRI density on the mast cell surface and the concentration of other mediators.
RESULTS
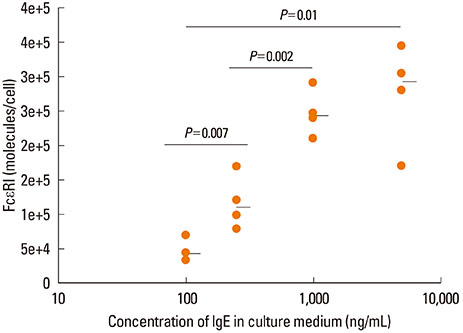
A clear correlation between IgE concentration in culture medium and the release of histamine upon activation was observed. It showed a bell-shaped dose response curve, with maximal response around an IgE-concentration of 250 ng/mL. Furthermore, the sensitivity of the mast cells and surface density of FcepsilonRI on mast cell surface was also influenced by the IgE concentration in the culture medium.
CONCLUSIONS
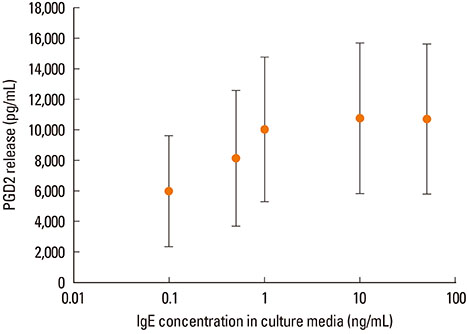
IgE in the culture medium during the last 10 days of mast cell maturation influences the release of the preformed mediator histamine after mast cell activation and the density of FcepsilonRI on the mast cell surface. The release of the de novo synthetized mediator prostaglandin D2 and the expression of chymase and tryptase are not influenced by IgE in culture medium.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Immune Characterization of Bone Marrow-Derived Models of Mucosal and Connective Tissue Mast Cells
Sara Benedé, Evan Cody, Charuta Agashe, M. Cecilia Berin
Allergy Asthma Immunol Res. 2018;10(3):268-277. doi: 10.4168/aair.2018.10.3.268.
Reference
-
1. Peavy RD, Metcalfe DD. Understanding the mechanisms of anaphylaxis. Curr Opin Allergy Clin Immunol. 2008; 8:310–315.2. Metcalfe DD, Peavy RD, Gilfillan AM. Mechanisms of mast cell signaling in anaphylaxis. J Allergy Clin Immunol. 2009; 124:639–646.3. Bansal G, Xie Z, Rao S, Nocka KH, Druey KM. Suppression of immunoglobulin E-mediated allergic responses by regulator of G protein signaling 13. Nat Immunol. 2008; 9:73–80.4. Bischoff SC. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat Rev Immunol. 2007; 7:93–104.5. Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997; 77:1033–1079.6. Siles RI, Hsieh FH. Allergy blood testing: a practical guide for clinicians. Cleve Clin J Med. 2011; 78:585–592.7. Baldacci S, Omenaas E, Oryszczyn MP. Allergy markers in respiratory epidemiology. Eur Respir J. 2001; 17:773–790.8. Borkowski TA, Jouvin MH, Lin SY, Kinet JP. Minimal requirements for IgE-mediated regulation of surface Fc epsilon RI. J Immunol. 2001; 167:1290–1296.9. King CL, Ottesen EA, Nutman TB. Cytokine regulation of antigen-driven immunoglobulin production in filarial parasite infections in humans. J Clin Invest. 1990; 85:1810–1815.10. Araujo MI, Lopes AA, Medeiros M, Cruz AA, Sousa-Atta L, Solé D, Carvalho EM. Inverse association between skin response to aeroallergens and Schistosoma mansoni infection. Int Arch Allergy Immunol. 2000; 123:145–148.11. Cooper PJ, Chico ME, Rodrigues LC, Ordonez M, Strachan D, Griffin GE, Nutman TB. Reduced risk of atopy among school-age children infected with geohelminth parasites in a rural area of the tropics. J Allergy Clin Immunol. 2003; 111:995–1000.12. Rodrigues LC, Newcombe PJ, Cunha SS, Alcantara-Neves NM, Genser B, Cruz AA, Simoes SM, Fiaccone R, Amorim L, Cooper PJ, Barreto ML. Social Change, Asthma and Allergy in Latin America. Early infection with Trichuris trichiura and allergen skin test reactivity in later childhood. Clin Exp Allergy. 2008; 38:1769–1777.13. van den Biggelaar AH, Rodrigues LC, van Ree R, van der Zee JS, Hoeksma-Kruize YC, Souverijn JH, Missinou MA, Borrmann S, Kremsner PG, Yazdanbakhsh M. Long-term treatment of intestinal helminths increases mite skin-test reactivity in Gabonese schoolchildren. J Infect Dis. 2004; 189:892–900.14. Andersen HB, Holm M, Hetland TE, Dahl C, Junker S, Schiøtz PO, Hoffmann HJ. Comparison of short term in vitro cultured human mast cells from different progenitors - peripheral blood-derived progenitors generate highly mature and functional mast cells. J Immunol Methods. 2008; 336:166–174.15. Holm M, Andersen HB, Hetland TE, Dahl C, Hoffmann HJ, Junker S, Schiøtz PO. Seven week culture of functional human mast cells from buffy coat preparations. J Immunol Methods. 2008; 336:213–221.16. Skov PS, Mosbech H, Norn S, Weeke B. Sensitive glass microfibre-based histamine analysis for allergy testing in washed blood cells. Results compared with conventional leukocyte histamine release assay. Allergy. 1985; 40:213–218.17. Sebaugh JL. Guidelines for accurate EC50/IC50 estimation. Pharm Stat. 2011; 10:128–134.18. Carosso A, Bugiani M, Migliore E, Antò JM, DeMarco R. Reference values of total serum IgE and their significance in the diagnosis of allergy in young European adults. Int Arch Allergy Immunol. 2007; 142:230–238.19. Woszczek G, Kowalski ML, Borowiec M. Association of asthma and total IgE levels with human leucocyte antigen-DR in patients with grass allergy. Eur Respir J. 2002; 20:79–85.20. Bazaral M, Hamburger RN. Standardization and stability of immunoglobulin E (IgE). J Allergy Clin Immunol. 1972; 49:189–191.21. Fishbein AB, Fuleihan RL. The hygiene hypothesis revisited: does exposure to infectious agents protect us from allergy? Curr Opin Pediatr. 2012; 24:98–102.22. Levin ME, Le Souëf PN, Motala C. Total IgE in urban Black South African teenagers: the influence of atopy and helminth infection. Pediatr Allergy Immunol. 2008; 19:449–454.23. Cooper PJ, Alexander N, Moncayo AL, Benitez SM, Chico ME, Vaca MG, Griffin GE. Environmental determinants of total IgE among school children living in the rural tropics: importance of geohelminth infections and effect of anthelmintic treatment. BMC Immunol. 2008; 9:33.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In Vitro Culture of Mast Cells from Human Umbilical Cord Blood Cells
- Effect of Interleukin-9 on Cell Proliferation and Histamine Release of Cord Blood-derived Human Mast Cells
- Evaluation of the MAST CLA Assay System for Measuring Total IgE: Comparison with the Pharmacia CAP System
- Role of Mast Cells in Allergic Inflammation and Innate Immunity
- Toll‐Like Receptor 7 (TLR7) Mediated Transcriptomic Changes on Human Mast Cells