Allergy Asthma Immunol Res.
2014 Nov;6(6):558-566. 10.4168/aair.2014.6.6.558.
The Serine Protease Inhibitor, 4-(2-aminoethyl) Benzene Sulfonyl Fluoride Hydrochloride, Reduces Allergic Inflammation in a House Dust Mite Allergic Rhinitis Mouse Model
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, The Catholic University of Korea, College of Medicine, Seoul, Korea. kshent@catholic.ac.kr
- KMID: 2260179
- DOI: http://doi.org/10.4168/aair.2014.6.6.558
Abstract
- PURPOSE
Serine protease inhibitors are involved in immune development, anti-inflammatory mechanisms, and tissue repair. In the present study, the serine protease inhibitor 4-(2-aminoethyl) benzene sulfonyl fluoride hydrochloride (AEBSF) was evaluated for its prophylactic and therapeutic applications in a mouse model of allergic rhinitis (AR).
METHODS
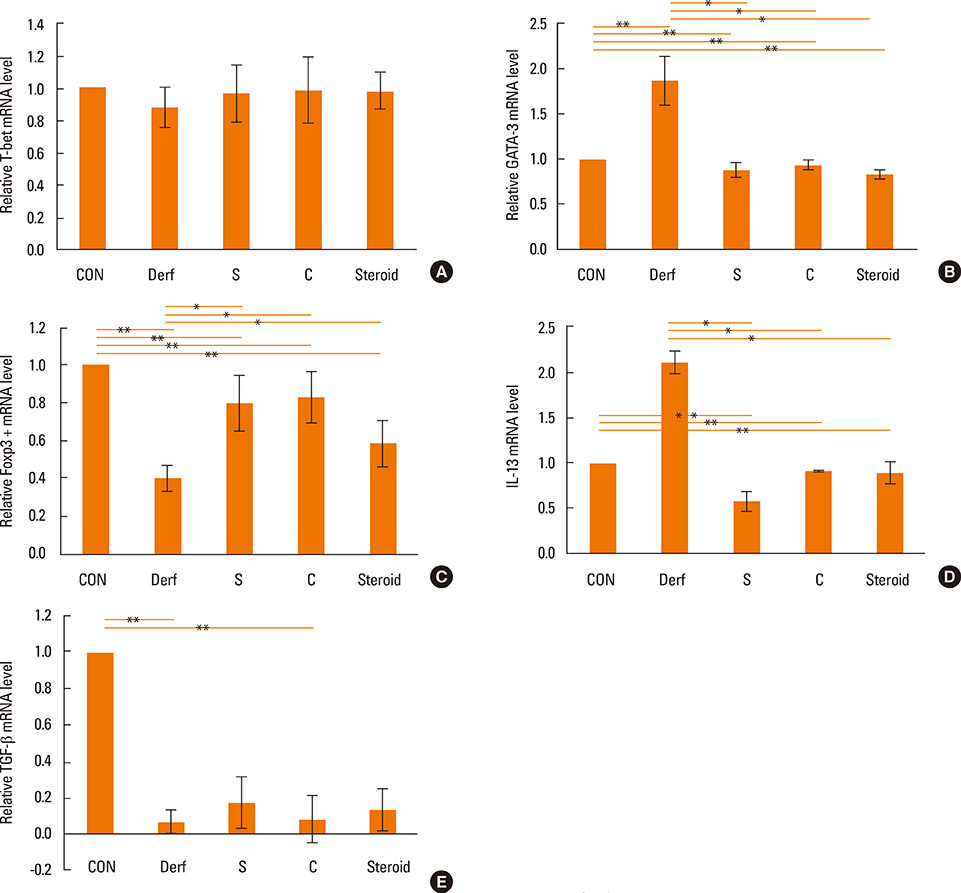
BALB/c mice were divided into 5 groups: contol (CON), Dermatophagoides farinae (Derf), AR mice treated with AEBSF before sensitization (S), AR mice treated with AEBSF after challenge (C), and steroid groups. Derf was used as an allergen. AEBSF was administered before S or after C. Allergic symptom scores, eosinophil counts, proteolytic activity, interferon-gamma, interleukin (IL)-10 levels and serum Derf-specific IgE levels were measured. T-bet, GATA-3, Foxp3, IL-13, and transforming growth factor (TGF)-beta mRNA levels were determined using real-time polymerase chain reaction. CD4+CD25+Foxp3+ T cells were assessed using flow cytometry.
RESULTS
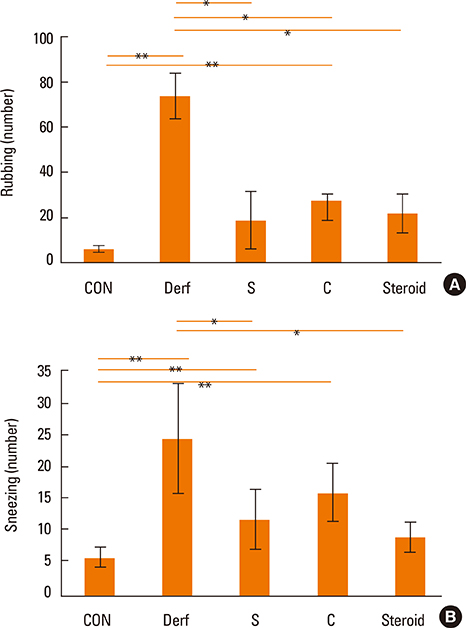
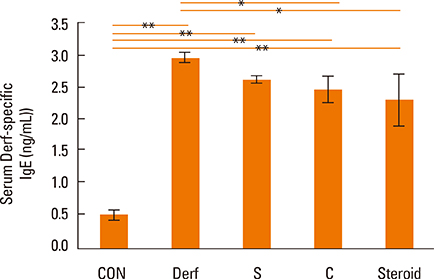
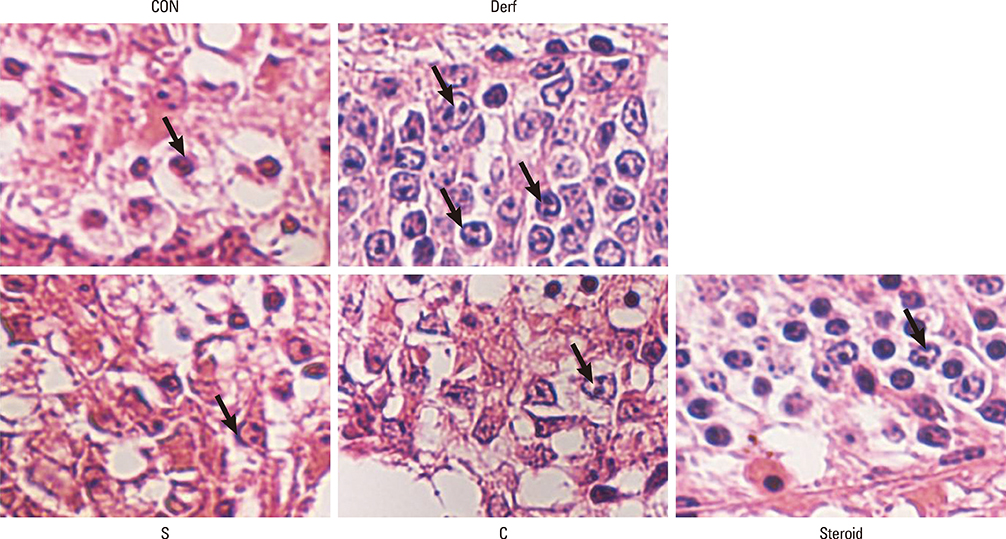
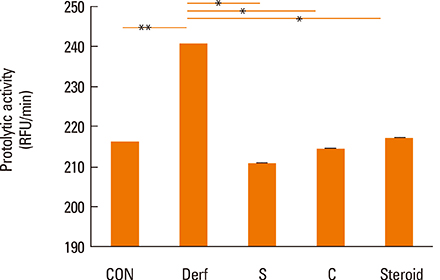
Symptom scores, serum Derf-specific IgE levels, GATA-3 mRNA levels, IL-13 mRNA levels, and tissue eosinophil counts decreased in both the S and C groups (P<0.05). Additionally, the percentage of CD4+CD25+Foxp3+ T cells, IL-10 levels, and Foxp3 mRNA levels increased in the S and C groups compared with those in the Derf group (P<0.05). AEBSF treatment decreased the proteolytic activity in the S and C groups (P<0.05).
CONCLUSIONS
Prophylactic and therapeutic treatment with AEBSF significantly reduces allergic airway inflammation and can induce regulatory T cells in a murine model of AR.
Keyword
MeSH Terms
-
Animals
Benzene*
Dermatophagoides farinae
Eosinophils
Flow Cytometry
Fluorides*
Immunoglobulin E
Inflammation*
Interferon-gamma
Interleukin-10
Interleukin-13
Interleukins
Mice*
Models, Animal
Pyroglyphidae*
Real-Time Polymerase Chain Reaction
Rhinitis*
RNA, Messenger
Serine Proteases*
Serine Proteinase Inhibitors
T-Lymphocytes
T-Lymphocytes, Regulatory
Transforming Growth Factors
Benzene
Fluorides
Immunoglobulin E
Interferon-gamma
Interleukin-10
Interleukin-13
Interleukins
RNA, Messenger
Serine Proteases
Serine Proteinase Inhibitors
Transforming Growth Factors
Figure
Reference
-
1. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canonica GW, van Weel C, Agache I, Aït-Khaled N, Bachert C, Blaiss MS, Bonini S, Boulet LP, Bousquet PJ, Camargos P, Carlsen KH, Chen Y, Custovic A, Dahl R, Demoly P, Douagui H, Durham SR, van Wijk RG, Kalayci O, Kaliner MA, Kim YY, Kowalski ML, Kuna P, Le LT, Lemiere C, Li J, Lockey RF, Mavale-Manuel S, Meltzer EO, Mohammad Y, Mullol J, Naclerio R, O'Hehir RE, Ohta K, Ouedraogo S, Palkonen S, Papadopoulos N, Passalacqua G, Pawankar R, Popov TA, Rabe KF, Rosado-Pinto J, Scadding GK, Simons FE, Toskala E, Valovirta E, van Cauwenberge P, Wang DY, Wickman M, Yawn BP, Yorgancioglu A, Yusuf OM, Zar H, Annesi-Maesano I, Bateman ED, Ben Kheder A, Boakye DA, Bouchard J, Burney P, Busse WW, Chan-Yeung M, Chavannes NH, Chuchalin A, Dolen WK, Emuzyte R, Grouse L, Humbert M, Jackson C, Johnston SL, Keith PK, Kemp JP, Klossek JM, Larenas-Linnemann D, Lipworth B, Malo JL, Marshall GD, Naspitz C, Nekam K, Niggemann B, Nizankowska-Mogilnicka E, Okamoto Y, Orru MP, Potter P, Price D, Stoloff SW, Vandenplas O, Viegi G, Williams D. World Health Organization. GA(2)LEN. AllerGen. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008; 63:Suppl 86. 8–160.2. Allen M, Heinzmann A, Noguchi E, Abecasis G, Broxholme J, Ponting CP, Bhattacharyya S, Tinsley J, Zhang Y, Holt R, Jones EY, Lench N, Carey A, Jones H, Dickens NJ, Dimon C, Nicholls R, Baker C, Xue L, Townsend E, Kabesch M, Weiland SK, Carr D, von Mutius E, Adcock IM, Barnes PJ, Lathrop GM, Edwards M, Moffatt MF, Cookson WO. Positional cloning of a novel gene influencing asthma from chromosome 2q14. Nat Genet. 2003; 35:258–263.3. Mao XQ, Shirakawa T, Yoshikawa T, Yoshikawa K, Kawai M, Sasaki S, Enomoto T, Hashimoto T, Furuyama J, Hopkin JM, Morimoto K. Association between genetic variants of mast-cell chymase and eczema. Lancet. 1996; 348:581–583.4. Smith PK, Harper JI. Serine proteases, their inhibitors and allergy. Allergy. 2006; 61:1441–1447.5. Izakovicova Holla L, Kanková K, Znojil V. Haplotype analysis of the NADPH oxidase p22 phox gene in patients with bronchial asthma. Int Arch Allergy Immunol. 2009; 148:73–80.6. Ishizaki M, Tanaka H, Kajiwara D, Toyohara T, Wakahara K, Inagaki N, Nagai H. Nafamostat mesilate, a potent serine protease inhibitor, inhibits airway eosinophilic inflammation and airway epithelial remodeling in a murine model of allergic asthma. J Pharmacol Sci. 2008; 108:355–363.7. Saw S, Kale SL, Arora N. Serine protease inhibitor attenuates ovalbumin induced inflammation in mouse model of allergic airway disease. PLoS One. 2012; 7:e41107.8. Nabe T, Ikedo A, Hosokawa F, Kishima M, Fujii M, Mizutani N, Yoshino S, Ishihara K, Akiba S, Chaplin DD. Regulatory role of antigen-induced interleukin-10, produced by CD4(+) T cells, in airway neutrophilia in a murine model for asthma. Eur J Pharmacol. 2012; 677:154–162.9. Zhou C, Kang XD, Chen Z. A synthetic Toll-like receptor 2 ligand decreases allergic immune responses in a mouse rhinitis model sensitized to mite allergen. J Zhejiang Univ Sci B. 2008; 9:279–285.10. Wang W, Zhu Z, Zhu B, Ma Z. Peroxisome proliferator-activated receptor-gamma agonist induces regulatory T cells in a murine model of allergic rhinitis. Otolaryngol Head Neck Surg. 2011; 144:506–513.11. Cho SH, Oh SY, Zhu Z, Lee J, Lane AP. Spontaneous eosinophilic nasal inflammation in a genetically-mutant mouse: comparative study with an allergic inflammation model. PLoS One. 2012; 7:e35114.12. Takahashi Y, Kagawa Y, Izawa K, Ono R, Akagi M, Kamei C. Effect of histamine H4 receptor antagonist on allergic rhinitis in mice. Int Immunopharmacol. 2009; 9:734–738.13. Minty A, Chalon P, Derocq JM, Dumont X, Guillemot JC, Kaghad M, Labit C, Leplatois P, Liauzun P, Miloux B, Minty C, Casellas P, Loison G, Lupker J, Shire D, Ferrara P, Caput D. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993; 362:248–250.14. Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000; 100:655–669.15. Yamashita M, Ukai-Tadenuma M, Miyamoto T, Sugaya K, Hosokawa H, Hasegawa A, Kimura M, Taniguchi M, DeGregori J, Nakayama T. Essential role of GATA3 for the maintenance of type 2 helper T (Th2) cytokine production and chromatin remodeling at the Th2 cytokine gene loci. J Biol Chem. 2004; 279:26983–26990.16. Fields ML, Hondowicz BD, Metzgar MH, Nish SA, Wharton GN, Picca CC, Caton AJ, Erikson J. CD4+ CD25+ regulatory T cells inhibit the maturation but not the initiation of an autoantibody response. J Immunol. 2005; 175:4255–4264.17. Ko K, Yamazaki S, Nakamura K, Nishioka T, Hirota K, Yamaguchi T, Shimizu J, Nomura T, Chiba T, Sakaguchi S. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exp Med. 2005; 202:885–891.18. Marino R, Thuraisingam T, Camateros P, Kanagaratham C, Xu YZ, Henri J, Yang J, He G, Ding A, Radzioch D. Secretory leukocyte protease inhibitor plays an important role in the regulation of allergic asthma in mice. J Immunol. 2011; 186:4433–4442.19. Wenzel SE, Fowler AA 3rd, Schwartz LB. Activation of pulmonary mast cells by bronchoalveolar allergen challenge. In vivo release of histamine and tryptase in atopic subjects with and without asthma. Am Rev Respir Dis. 1988; 137:1002–1008.20. Simpson JL, Scott RJ, Boyle MJ, Gibson PG. Differential proteolytic enzyme activity in eosinophilic and neutrophilic asthma. Am J Respir Crit Care Med. 2005; 172:559–565.21. Inoue K, Takano H, Yoshikawa T. Protease-antiprotease imbalance in inflammatory diseases in the lung. Chest. 2005; 128:1069.22. Saw S, Kale SL, Arora N. Serine protease inhibitor attenuates ovalbumin induced inflammation in mouse model of allergic airway disease. PLoS One. 2012; 7:e41107.23. Oh SW, Pae CI, Lee DK, Jones F, Chiang GK, Kim HO, Moon SH, Cao B, Ogbu C, Jeong KW, Kozu G, Nakanishi H, Kahn M, Chi EY, Henderson WR Jr. Tryptase inhibition blocks airway inflammation in a mouse asthma model. J Immunol. 2002; 168:1992–2000.24. Winton HL, Wan H, Cannell MB, Thompson PJ, Garrod DR, Stewart GA, Robinson C. Class specific inhibition of house dust mite proteinases which cleave cell adhesion, induce cell death and which increase the permeability of lung epithelium. Br J Pharmacol. 1998; 124:1048–1059.25. Sakaguchi S, Wing K, Miyara M. Regulatory T cells - a brief history and perspective. Eur J Immunol. 2007; 37:Suppl 1. S116–S123.26. Rudensky AY. Regulatory T cells and Foxp3. Immunol Rev. 2011; 241:260–268.27. Sakaguchi S, Wing K, Miyara M. Regulatory T cells - a brief history and perspective. Eur J Immunol. 2007; 37:Suppl 1. S116–S123.28. Valencia X, Lipsky PE. CD4+CD25+FoxP3+ regulatory T cells in autoimmune diseases. Nat Clin Pract Rheumatol. 2007; 3:619–626.29. Ling EM, Smith T, Nguyen XD, Pridgeon C, Dallman M, Arbery J, Carr VA, Robinson DS. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004; 363:608–615.30. Waldmann H, Chen TC, Graca L, Adams E, Daley S, Cobbold S, Fairchild PJ. Regulatory T cells in transplantation. Semin Immunol. 2006; 18:111–119.31. Graca L, Le Moine A, Cobbold SP, Waldmann H. Dominant transplantation tolerance. Opinion. Curr Opin Immunol. 2003; 15:499–506.32. Wood KJ, Bushell A, Hester J. Regulatory immune cells in transplantation. Nat Rev Immunol. 2012; 12:417–430.33. Cottrez F, Hurst SD, Coffman RL, Groux H. T regulatory cells 1 inhibit a Th2-specific response in vivo. J Immunol. 2000; 165:4848–4853.34. Navarro S, Cossalter G, Chiavaroli C, Kanda A, Fleury S, Lazzari A, Cazareth J, Sparwasser T, Dombrowicz D, Glaichenhaus N, Julia V. The oral administration of bacterial extracts prevents asthma via the recruitment of regulatory T cells to the airways. Mucosal Immunol. 2011; 4:53–65.35. Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001; 27:20–21.36. Shevach EM. Mechanisms of Foxp3+ T regulatory cell-mediated suppression. Immunity. 2009; 30:636–645.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Distribution of House Dust Mites in the Bedroom of Patients with Allergic Rhinitis in Pusan Area
- Principles and Application of Mouse Model of Allergic Rhinitis
- Understanding the Mouse Model of Respiratory Allergic Diseases
- Allergic Rhinitis Mouse Model
- House Dust Mite Allergic Rhinitis Model in C57BL/6 Mice