Korean J Hematol.
2011 Sep;46(3):169-174. 10.5045/kjh.2011.46.3.169.
Recent advances in the path toward the cure for chronic myeloid leukemia
- Affiliations
-
- 1Department of Hematology, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea. dwkim@catholic.ac.kr
- KMID: 2251999
- DOI: http://doi.org/10.5045/kjh.2011.46.3.169
Abstract
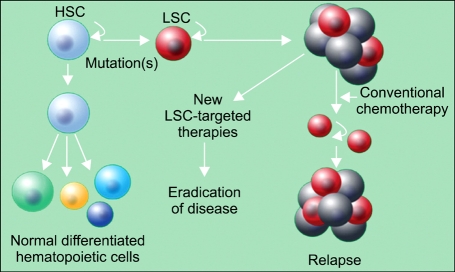
- Through the phase 3 International Randomized Study of Interferon vs. STI571 (IRIS) trial, imatinib emerged as the standard treatment for chronic myeloid leukemia (CML) and has successfully prolonged the duration of both the chronic phase (CP) and the disease-free state. The majority of newly diagnosed patients treated for CP-CML achieve a complete cytogenetic response (CCyR), and over time, most of these eventually achieve major molecular responses (MMRs) and even complete molecular responses (CMRs). In ongoing phase 3 randomized trials of second-generation tyrosine kinase inhibitors (TKIs), nilotinib and dasatinib have been found to have superior efficacies in helping achieve cytogenetic and molecular responses, including MMRs and CMRs. However, only the MMR rate was significantly higher in bosutinib compared with the imatinib control, but not in CCyR rate. Current reports of imatinib discontinuation suggested that achieving CMR is an important prerequisite for CML to be cured. Recent data from the STIM (Stop Imatinib) trial showed that imatinib can be successfully discontinued in patients who achieve a certain level of CMR. Standardized real-time quantitative reverse transcriptase-polymerase chain reaction (RQ-PCR) assays have been available in routine clinical practice, and efforts are being focused on achieving higher sensitivity and optimizing the time of imatinib discontinuation. Although very few patients are cured by administration of only Bcr-Abl TKIs, including imatinib and second-generation TKIs, current advances may eventually make this possible. This report summarizes the detailed clinical data obtained in the DASISION, ENESTnd, and BELA studies and discusses high-sensitivity detection methods and future therapeutic strategies.
MeSH Terms
-
Aniline Compounds
Benzamides
Cytogenetics
Humans
Interferons
Leukemia, Myelogenous, Chronic, BCR-ABL Positive
Nitriles
Piperazines
Polymerase Chain Reaction
Protein-Tyrosine Kinases
Pyrimidines
Quinolines
Thiazoles
Dasatinib
Imatinib Mesylate
Aniline Compounds
Benzamides
Interferons
Nitriles
Piperazines
Protein-Tyrosine Kinases
Pyrimidines
Quinolines
Thiazoles
Figure
Reference
-
1. Hochhaus A, O'Brien SG, Guilhot F, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009; 23:1054–1061. PMID: 19282833.
Article2. Hughes TP, Kaeda J, Branford S, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003; 349:1423–1432. PMID: 14534335.
Article3. Branford S, Seymour JF, Grigg A, et al. BCR-ABL messenger RNA levels continue to decline in patients with chronic phase chronic myeloid leukemia treated with imatinib for more than 5 years and approximately half of all first-line treated patients have stable undetectable BCR-ABL using strict sensitivity criteria. Clin Cancer Res. 2007; 13:7080–7085. PMID: 18056186.
Article4. de Lavallade H, Apperley JF, Khorashad JS, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008; 26:3358–3363. PMID: 18519952.
Article5. Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009; 27:6041–6051. PMID: 19884523.
Article6. Kantarjian H, O'Brien S, Shan J, et al. Cytogenetic and molecular responses and outcome in chronic myelogenous leukemia: need for new response definitions? Cancer. 2008; 112:837–845. PMID: 18085610.7. Quintás-Cardama A, Kantarjian H, Jones D, et al. Delayed achievement of cytogenetic and molecular response is associated with increased risk of progression among patients with chronic myeloid leukemia in early chronic phase receiving high-dose or standard-dose imatinib therapy. Blood. 2009; 113:6315–6321. PMID: 19369233.
Article8. Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010; 362:2251–2259. PMID: 20525993.
Article9. Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010; 362:2260–2270. PMID: 20525995.
Article10. Brümmendorf T, Gambacorti-Passerini C, Cortes J, et al. The BELA trial: bosutinib versus imatinib in patients with chronic phase chronic myeloid leukemia; 18-month follow-up. Haematologica. 2011; 96(Suppl 2):204. (abst 0485). PMID: 21071498.11. Kantarjian HM, Hochhaus A, Saglio G, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol. 2011; 12:841–851. PMID: 21856226.
Article12. Hochhaus A, Shah N, Cortes J, et al. Efficacy and safety of dasatinib compared with imatinib in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP): minimum 24-month follow-up from the DASISION trial. Haematologica. 2011; 96(Suppl 2):422. (abst 1011).13. Goh HG, Kim YJ, Kim DW, et al. Previous best responses can be re-achieved by resumption after imatinib discontinuation in patients with chronic myeloid leukemia: implication for intermittent imatinib therapy. Leuk Lymphoma. 2009; 50:944–951. PMID: 19479613.
Article14. Koskenvesa P, Mustjoki S, Rasanen A, et al. Imatinib discontinuation following a major molecular response: impact of interferon alpha and leukemia stem cell burden (The STOP Study). Blood. 2008; 112:738. (abst 2121).
Article15. Kuwabara A, Babb A, Ibrahim A, et al. Poor outcome after reintroduction of imatinib in patients with chronic myeloid leukemia who interrupt therapy on account of pregnancy without having achieved an optimal response. Blood. 2010; 116:1014–1016. PMID: 20705771.
Article16. Mahon FX, Réa D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010; 11:1029–1035. PMID: 20965785.
Article17. Rea D, Rousselot P, Nicolini F, et al. Cessation of dasatinib or nilotinib therapy in chronic-phase chronic myeloid leukaemia patients with sustained complete molecular responses. Haematologica. 2011; 96(Suppl 2):423–424. (abst 1014).18. Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006; 108:1809–1820. PMID: 16709930.
Article19. Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006; 108:28–37. PMID: 16522812.
Article20. Branford S, Fletcher L, Cross NC, et al. Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood. 2008; 112:3330–3338. PMID: 18684859.
Article21. Hughes TP, Hochhaus A, Saglio G, et al. ENESTnd update: continued superiority of nilotinib versus imatinib in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP). Blood. 2010; 116:94–95. (abst 207).
Article22. Choi SY, Goh HG, Kim D, et al. Dynamics of molecular response to standard-dose imatinib in new CP chronic myeloid leukemia patients after achieving CMR4.0. Haematologica. 2011; 96(Suppl 2):62. (abst 0153). PMID: 20952518.23. Sawyers CL. Chronic myeloid leukemia. N Engl J Med. 1999; 340:1330–1340. PMID: 10219069.
Article24. Mauro MJ, Druker BJ, Maziarz RT. Divergent clinical outcome in two CML patients who discontinued imatinib therapy after achieving a molecular remission. Leuk Res. 2004; 28(Suppl 1):S71–S73. PMID: 15036945.
Article25. Ross DM, Branford S, Seymour JF, et al. Patients with chronic myeloid leukemia who maintain a complete molecular response after stopping imatinib treatment have evidence of persistent leukemia by DNA PCR. Leukemia. 2010; 24:1719–1724. PMID: 20811403.
Article26. Goh HG, Lin M, Fukushima T, et al. Sensitive quantitation of minimal residual disease in chronic myeloid leukemia using nanofluidic digital polymerase chain reaction assay. Leuk Lymphoma. 2011; 52:896–904. PMID: 21338281.
Article27. Graham SM, Jørgensen HG, Allan E, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002; 99:319–325. PMID: 11756187.
Article28. Chomel JC, Bonnet ML, Sorel N, et al. Leukemic stem cell persistency in chronic myeloid leukemia patients with sustained undetectable molecular residual disease. Blood. 2011; [Epub ahead of print].29. Dierks C, Beigi R, Guo GR, et al. Expansion of Bcr-Abl-positive leukemic stem cells is dependent on Hedgehog pathway activation. Cancer Cell. 2008; 14:238–249. PMID: 18772113.
Article30. Zhao C, Blum J, Chen A, et al. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007; 12:528–541. PMID: 18068630.31. Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003; 423:255–260. PMID: 12714970.
Article32. Jamieson CH, Ailles LE, Dylla SJ, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004; 351:657–667. PMID: 15306667.
Article33. Medina V, Calvo MB, Díaz-Prado S, Espada J. Hedgehog signalling as a target in cancer stem cells. Clin Transl Oncol. 2009; 11:199–207. PMID: 19380296.
Article34. Kuroda J, Taniwaki M. Life and death of leukemic cells under Bcr-Abl signaling control. Curr Cancer Ther Rev. 2009; 5:303–309.
Article35. Essers MA, Offner S, Blanco-Bose WE, et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009; 458:904–908. PMID: 19212321.36. Jørgensen HG, Allan EK, Jordanides NE, Mountford JC, Holyoake TL. Nilotinib exerts equipotent antiproliferative effects to imatinib and does not induce apoptosis in CD34+ CML cells. Blood. 2007; 109:4016–4019. PMID: 17213283.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Leukemia Cutis in Myelodysplastic Syndrome Evolving into An Atypical Chronic Myeloid Leukemia
- Acute myeloid leukemia arising from chronic myelomonocytic leukemia during hypomethylating therapy
- Acute Myeloid Leukemia after Chemotherapy for Osteosarcoma: A Case Report
- A Case of Acute Myeloid Leukemia Concurrent With Untreated Chronic Lymphocytic Leukemia
- Two Cases of Chronic Myeloid Leukemia in Lymphoid Blast Phase Presented as Philadelphia-Positive Acute Lymphoblastic Leukemia