Korean J Hematol.
2012 Mar;47(1):44-52. 10.5045/kjh.2012.47.1.44.
Factors influencing lymphocyte reconstitution after allogeneic hematopoietic stem cell transplantation in children
- Affiliations
-
- 1Division of Pediatric Hematology/Oncology, Department of Pediatrics, Asan Medical Center Children's Hospital, University of Ulsan College of Medicine, Seoul, Korea. jjseo@amc.seoul.kr
- KMID: 2251970
- DOI: http://doi.org/10.5045/kjh.2012.47.1.44
Abstract
- BACKGROUND
Immune reconstitution (IR) after hematopoietic stem cell transplantation (HSCT) reduces transplantation-related complications such as infection and improves HSCT outcomes.
METHODS
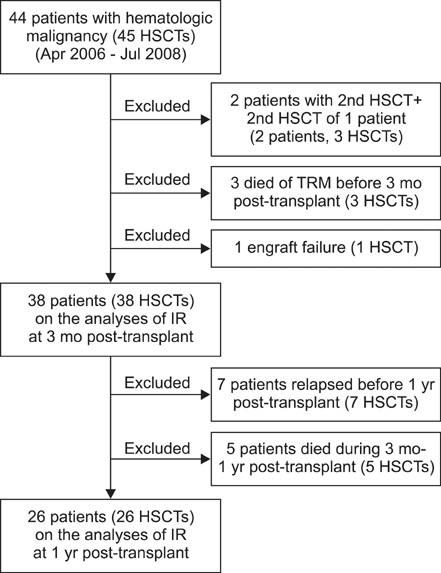
We retrospectively analyzed IR of lymphocyte subpopulations in 38 pediatric patients for hematologic malignant diseases after allogeneic HSCT from April 2006 to July 2008. T-cell-, B-cell-, and natural killer (NK) cell-associated antigens were assayed in peripheral blood by flow cytometry analysis of 5 lymphocyte subsets, CD3+, CD3+/CD4+, CD4+/CD8+, CD16+/CD56+, and CD19+, before and 3 and 12 months after transplantation.
RESULTS
Reconstitutions of CD16+/CD56+ and CD3+/CD8+ lymphocytes were achieved rapidly, whereas that of CD3+/CD19+ lymphocytes occurred later. Age was not related to reconstitution of any lymphocyte subset. Total body irradiation (TBI) and anti-thymocyte globulin (ATG) administration were related to delayed reconstitution of total lymphocytes and CD3+ lymphocytes, respectively. Reconstitutions of CD3+/CD4+ lymphocytes and CD3+/CD8+ lymphocytes were significantly delayed in patients who received umbilical cord blood stem cells. In patients with chronic graft-versus-host disease (cGVHD), recovery of the total lymphocyte count and CD19+ lymphocytes at 3 months post-transplant were significantly delayed. However, acute GVHD (aGVHD) and cytomegalovirus (CMV) reactivation did not influence the IR of any lymphocyte subset. Further, delayed reconstitution of lymphocyte subsets did not correspond to inferior survival outcomes in this study.
CONCLUSION
We observed that some lymphocyte reconstitutions after HSCT were influenced by the stem cell source and preparative regimens. However, delayed CD19+ lymphocyte reconstitution may be associated with cGVHD.
MeSH Terms
Figure
Reference
-
1. Gross TG, Egeler RM, Smith FO. Pediatric hematopoietic stem cell transplantation. Hematol Oncol Clin North Am. 2001. 15:795–808.
Article2. Parkman R, Weinberg KI. Applebaum FR, Forman SJ, Negrin RS, Blume KG, editors. Immune reconstitution following hematopoietic cell transplantation. Thomas hematopoietic stem cell transplantation. 2008. 4th ed. West Sussex, UK: John Wiley & Sons;222–232.
Article3. Storek J, Geddes M, Khan F, et al. Reconstitution of the immune system after hematopoietic stem cell transplantation in humans. Semin Immunopathol. 2008. 30:425–437.
Article4. Lum LG. The kinetics of immune reconstitution after human marrow transplantation. Blood. 1987. 69:369–380.
Article5. Verma UN, Mazumder A. Immune reconstitution following bone marrow transplantation. Cancer Immunol Immunother. 1993. 37:351–360.
Article6. Storek J, Witherspoon RP, Storb R. T cell reconstitution after bone marrow transplantation into adult patients does not resemble T cell development in early life. Bone Marrow Transplant. 1995. 16:413–425.7. Williams KM, Gress RE. Immune reconstitution and implications for immunotherapy following haematopoietic stem cell transplantation. Best Pract Res Clin Haematol. 2008. 21:579–596.
Article8. Storek J, Zhao Z, Lin E, et al. Recovery from and consequences of severe iatrogenic lymphopenia (induced to treat autoimmune diseases). Clin Immunol. 2004. 113:285–298.
Article9. Petersen SL, Ryder LP, Bjork P, et al. A comparison of T-, B- and NK-cell reconstitution following conventional or nonmyeloablative conditioning and transplantation with bone marrow or peripheral blood stem cells from human leucocyte antigen identical sibling donors. Bone Marrow Transplant. 2003. 32:65–72.
Article10. Hollander GA. Lymphoid reconstitution following hematopoietic stem cell transplantation. Of mice and men: progress made in HSCT immunobiology. Semin Immunopathol. 2008. 30:369–370.
Article11. Kalwak K, Gorczynska E, Toporski J, et al. Immune reconstitution after haematopoietic cell transplantation in children: immunophenotype analysis with regard to factors affecting the speed of recovery. Br J Haematol. 2002. 118:74–89.
Article12. Baron F, Baker JE, Storb R, et al. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2004. 104:2254–2262.
Article13. Kim DH, Sohn SK, Won DI, Lee NY, Suh JS, Lee KB. Rapid helper T-cell recovery above 200×106/L at 3 months correlates to successful transplant outcomes after allogeneic stem cell transplantation. Bone Marrow Transplant. 2006. 37:1119–1128.
Article14. Krause H, Hebart H, Jahn G, Muller CA, Einsele H. Screening for CMV-specific T cell proliferation to identify patients at risk of developing late onset CMV disease. Bone Marrow Transplant. 1997. 19:1111–1116.
Article15. Novitzky N, Davison GM, Hale G, Waldmann H. Immune reconstitution at 6 months following T-cell depleted hematopoietic stem cell transplantation is predictive for treatment outcome. Transplantation. 2002. 74:1551–1559.
Article16. Storek J, Espino G, Dawson MA, Storer B, Flowers ME, Maloney DG. Low B-cell and monocyte counts on day 80 are associated with high infection rates between days 100 and 365 after allogeneic marrow transplantation. Blood. 2000. 96:3290–3293.
Article17. Geddes M, Storek J. Immune reconstitution following hematopoietic stem-cell transplantation. Best Pract Res Clin Haematol. 2007. 20:329–348.
Article18. Foot AB, Potter MN, Donaldson C, et al. Immune reconstitution after BMT in children. Bone Marrow Transplant. 1993. 11:7–13.19. Kook H, Goldman F, Padley D, et al. Reconstruction of the immune system after unrelated or partially matched T-cell-depleted bone marrow transplantation in children: immunophenotypic analysis and factors affecting the speed of recovery. Blood. 1996. 88:1089–1097.
Article20. de Vries E, van Tol MJ, van den Bergh RL, et al. Reconstitution of lymphocyte subpopulations after paediatric bone marrow transplantation. Bone Marrow Transplant. 2000. 25:267–275.
Article21. Moretta A, Maccario R, Fagioli F, et al. Analysis of immune reconstitution in children undergoing cord blood transplantation. Exp Hematol. 2001. 29:371–379.
Article22. Comans-Bitter WM, de Groot R, van den Beemd R, et al. Immunophenotyping of blood lymphocytes in childhood: Reference values for lymphocyte subpopulations. J Pediatr. 1997. 130:388–393.
Article23. Kim JS, Lee WK, Suh JS, et al. T and B cell changes with aging. Korean J Clin Pathol. 2001. 21:135–140.24. Favrot M, Janossy G, Tidman N, et al. T cell regeneration after allogeneic bone marrow transplantation. Clin Exp Immunol. 1983. 54:59–72.25. Mackall CL, Hakim FT, Gress RE. T-cell regeneration: all repertoires are not created equal. Immunol Today. 1997. 18:245–251.
Article26. Storek J, Joseph A, Dawson MA, Douek DC, Storer B, Maloney DG. Factors influencing T-lymphopoiesis after allogeneic hematopoietic cell transplantation. Transplantation. 2002. 73:1154–1158.
Article27. Small TN, Avigan D, Dupont B, et al. Immune reconstitution following T-cell depleted bone marrow transplantation: effect of age and posttransplant graft rejection prophylaxis. Biol Blood Marrow Transplant. 1997. 3:65–75.28. Small TN, Papadopoulos EB, Boulad F, et al. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999. 93:467–480.
Article29. Mackall CL, Fleisher TA, Brown MR, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995. 332:143–149.
Article30. Small TN. Kline RM, editor. Immune reconstitution in pediatric patients following hematopoietic stem-cell transplantation. Pediatric hematopoietic stem cell transplantation. 2006. New York, NY: Informa Healthcare;271–285.31. Ottinger HD, Beelen DW, Scheulen B, Schaefer UW, Grosse-Wilde H. Improved immune reconstitution after allotransplantation of peripheral blood stem cells instead of bone marrow. Blood. 1996. 88:2775–2779.
Article32. Giraud P, Thuret I, Reviron D, et al. Immune reconstitution and outcome after unrelated cord blood transplantation: a single paediatric institution experience. Bone Marrow Transplant. 2000. 25:53–57.
Article33. Niehues T, Rocha V, Filipovich AH, et al. Factors affecting lymphocyte subset reconstitution after either related or unrelated cord blood transplantation in children- a Eurocord analysis. Br J Haematol. 2001. 114:42–48.
Article34. Szabolcs P, Niedzwiecki D. Immune reconstitution after unrelated cord blood transplantation. Cytotherapy. 2007. 9:111–122.
Article35. Noel DR, Witherspoon RP, Storb R, et al. Does graft-versus-host disease influence the tempo of immunologic recovery after allogeneic human marrow transplantation? An observation on 56 long-term survivors. Blood. 1978. 51:1087–1105.
Article36. Weinberg K, Blazar BR, Wagner JE, et al. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood. 2001. 97:1458–1466.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immune reconstitution after allogeneic hematopoietic stem cell transplantation in children: a single institution study of 59 patients
- A case of pneumomediastinum combined with chronic graft-versus-host disease following allogeneic hematopoietic stem cell transplantation
- Allogeneic hematopoietic stem cell transplantation for myelodysplastic syndromes
- Comparison of Quality of Life of Autologous and Allogeneic Hematopoietic Stem Cell Transplantation Recipients
- Pediatric Allogeneic Hematopoietic Stem Cell Transplantation in Korea: April 2000: The Korean Society of Pediatric Hematology-Oncology