Korean J Androl.
2011 Aug;29(2):111-126. 10.5534/kja.2011.29.2.111.
Differential Expression of Proteins Related with Penile Apoptosis in a Rat after Cavernous Nerve Resection
- Affiliations
-
- 1Department of Urology, School of Medicine, Chungju Hospital, Konkuk University, Chungju, Korea.
- 2Department of Physiology, School of Medicine, Konkuk University, Chungju, Korea.
- 3National Research Laboratory of Regenerative Sexual Medicine and Department of Urology, College of Medicine, Inha University, Incheon, Korea.
- 4Department of Anatomy, School of Medicine, Konkuk University, Chungju, Korea.
- KMID: 2226464
- DOI: http://doi.org/10.5534/kja.2011.29.2.111
Abstract
- PURPOSE
The mechanism including changes of proteome within cavernosal tissue after cavernous nerve injury were not evaluated. We performed proteomics and functional analysis to identify proteins of penile corpus cavernosum whose expression was or was not altered by cavernous nerve resection (CNR).
MATERIALS AND METHODS
Using 8-week-old male WKY rats, sham and CNR operation under microscope were performed. After 8 weeks, penile tissues of sham and CNR group were harvested. We used 2-DE and MALDI-TOF/TOF (AB 4700) to identify of differently expressed penile proteins. 2-DE gels were stained with silver nitrate and the gels were analyzed with PDQuest.
RESULTS
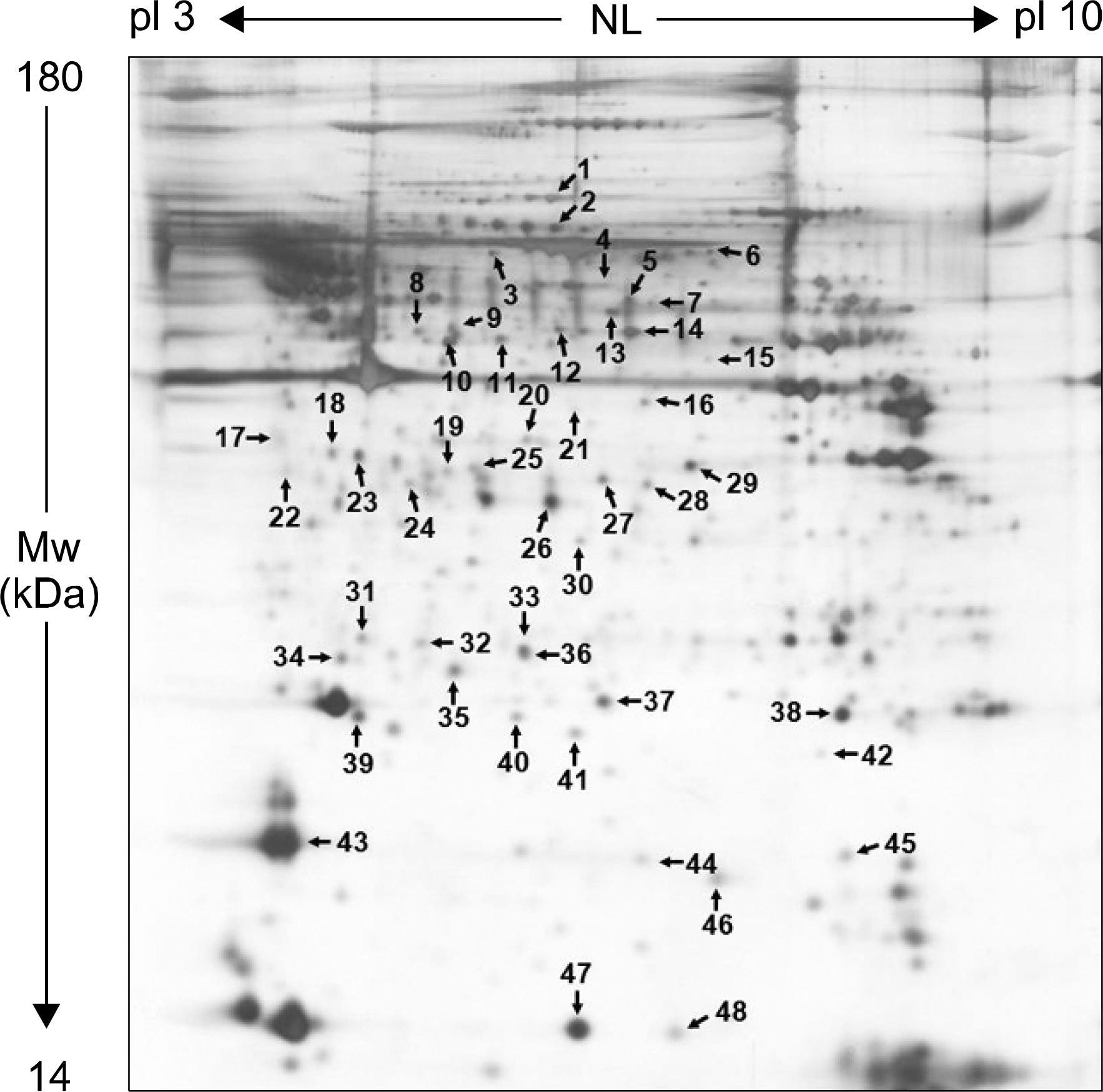
We isolated more than 950 proteins on silver-stained gels of whole protein extracts from normal rat penile corpus cavernous. Of these proteins, 48 prominent proteins were identified using MALDI-TOF/TOF. Protein characterization revealed that the most prominent penile corpus cavernous proteins were those with antioxidant, chaperone, or cytoskeletal structure. Moreover, 11 proteins having levels elevated by CNR were annexin proteins, endoplasmic reticulum protein 29, glutathione s-transferase w-1, and others. In addition, Rho-GDP dissociation inhibitor (RhoGDI), a regulator of Rho proteins, was also increased in CNR rats compared with sham-operated control rats.
CONCLUSIONS
The apoptotic signals observed in penile tissues was greatly increased in CNR rats than in sham-operated rats. These results suggest that RhoGDI is one of the proteins regulated by CNR in penile smooth muscle strips, and has a crucial role in the early stage of penile apoptosis.
Keyword
MeSH Terms
-
Animals
Apoptosis
Caves
Dissociative Disorders
Endoplasmic Reticulum
Erectile Dysfunction
Gels
Glutathione Transferase
Humans
Male
Muscle, Smooth
Proteins
Proteome
Proteomics
Rats
Rats, Inbred WKY
rho-Specific Guanine Nucleotide Dissociation Inhibitors
Salicylamides
Silver Nitrate
Gels
Glutathione Transferase
Proteins
Proteome
Salicylamides
Silver Nitrate
rho-Specific Guanine Nucleotide Dissociation Inhibitors
Figure
Reference
-
1). Burnett AL. Strategies to promote recovery of cavernous nerve function after radical prostatectomy. World J Urol. 2003; 20:337–42.
Article2). Olsson CA, Goluboff ET. Detection and treatment of prostate cancer: perspective of the urologist. J Urol. 1994; 152:1695–9.
Article3). Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982; 128:492–7.
Article4). Takenaka A, Murakami G, Matsubara A, Han SH, Fujisawa M. Variation in course of cavernous nerve with special reference to details of topographic relationships near prostatic apex: histologic study using male cadavers. Urology. 2005; 65:136–42.
Article5). Lepor H, Gregerman M, Crosby R, Mostofi FK, Walsh PC. Precise localization of the autonomic nerves from the pelvic plexus to the corpora cavernosa: a detailed anatomical study of the adult male pelvis. J Urol. 1985; 133:207–12.
Article6). Montorsi F, Briganti A, Salonia A, Rigatti P, Burnett AL. Current and future strategies for preventing and managing erectile dysfunction following radical prostatectomy. Eur Urol. 2004; 45:123–33.
Article7). Costello AJ, Brooks M, Cole OJ. Anatomical studies of the neurovascular bundle and cavernosal nerves. BJU Int. 2004; 94:1071–6.
Article8). Sezen SF, Burnett AL. Intracavernosal pressure monitoring in mice: responses to electrical stimulation of the cavernous nerve and to intracavernosal drug administration. J Androl. 2000; 21:311–5.9). Lysiak JJ, Yang SK, Klausner AP, Son H, Tuttle JB, Steers WD. Tadalafil increases Akt and extracellular signal-regulated kinase 1/2 activation, and prevents apoptotic cell death in the penis following denervation. J Urol. 2008; 179:779–85.
Article10). Mullerad M, Donohue JF, Li PS, Scardino PT, Mulhall JP. Functional sequelae of cavernous nerve injury in the rat: is there model dependency. J Sex Med. 2006; 3:77–83.
Article11). Klein LT, Miller MI, Buttyan R, Raffo AJ, Burchard M, Devris G, et al. Apoptosis in the rat penis after penile denervation. J Urol. 1997; 158:626–30.
Article12). User HM, Hairston JH, Zelner DJ, McKenna KE, McVary KT. Penile weight and cell subtype specific changes in a post-radical prostatectomy model of erectile dysfunction. J Urol. 2003; 169:1175–9.
Article13). Hu WL, Hu LQ, Song J, Li SW, Zheng XM, Cheng B, et al. Fibrosis of corpus cavernosum in animals following cavernous nerve ablation. Asian J Androl. 2004; 6:111–6.14). Carrier S, Zvara P, Nunes L, Kour NW, Rehman J, Lue TF. Regeneration of nitric oxide synthase-containing nerves after cavernous nerve neurotomy in the rat. J Urol. 1995; 153:1722–7.
Article15). Iacono F, Giannella R, Somma P, Manno G, Fusco F, Mirone V. Histological alterations in cavernous tissue after radical prostatectomy. J Urol. 2005; 173:1673–6.
Article16). Domes T, De Young L, O'Gorman DB, Gan BS, Bella AJ, Brock G. Is there a role for proteomics in Peyronie's disease? J Sex Med. 2007; 4:867–77.
Article17). Liu X, Gao X, Pang J, Zhang Y, Wang K, Fang Y, et al. Proteomic analysis of rat penile tissue in a model of erectile dysfunction after radical prostatectomy. BJU Int. 2007; 99:1500–5.
Article18). User HM, Zelner DJ, McKenna KE, McVary KT. Microarray analysis and description of SMR1 gene in rat penis in a post-radical prostatectomy model of erectile dysfunction. J Urol. 2003; 170:298–301.
Article19). Lee CK, Park HJ, So HH, Kim HJ, Lee KS, Choi WS, et al. Proteomic profiling and identification of cofilin responding to oxidative stress in vascular smooth muscle. Proteomics. 2006; 6:6455–75.
Article20). Lee CK, Han JS, Won KJ, Jung SH, Park HJ, Lee HM, et al. Diminished expression of dihydropteridine reductase is a potent biomarker for hypertensive vessels. Proteomics. 2009; 9:4851–8.
Article21). Tostes RC, Carneiro FS, Lee AJ, Giachini FR, Leite R, Osawa Y, et al. Cigarette smoking and erectile dysfunction: focus on NO bioavailability and ROS generation. J Sex Med. 2008; 5:1284–95.
Article22). Wolf BB, Green DR. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J Biol Chem. 1999; 274:20049–52.
Article23). Sasaki T, Takai Y. The Rho small G protein family-Rho GDI system as a temporal and spatial determinant for cytoskeletal control. Biochem Biophys Res Commun. 1998; 245:641–5.
Article24). Hoffman GR, Nassar N, Cerione RA. Structure of the Rho family GTP-binding protein Cdc42 in complex with the multifunctional regulator RhoGDI. Cell. 2000; 100:345–56.
Article25). Miñambres R, Guasch RM, Perez-Aragó A, Guerri C. The RhoA/ROCK-I/MLC pathway is involved in the ethanol-induced apoptosis by anoikis in astrocytes. J Cell Sci. 2006; 119:271–82.26). Harenberg A, Girkontaite I, Giehl K, Fischer KD. The Lsc RhoGEF mediates signaling from thromboxane A2 to actin polymerization and apoptosis in thymocytes. Eur J Immunol. 2005; 35:1977–86.
Article27). He H, Baldwin GS. Rho GTPases and p21-activated kinase in the regulation of proliferation and apoptosis by gastrins. Int J Biochem Cell Biol. 2008; 40:2018–22.
Article28). Alici B, Gümüstas MK, Ozkara H, Akkus E, Demirel G, Yencilek F, et al. Apoptosis in the erectile tissues of diabetic and healthy rats. BJU Int. 2000; 85:326–9.
Article29). Jin L, Liu T, Lagoda GA, Champion HC, Bivalacqua TJ, Burnett AL. Elevated RhoA/Rho-kinase activity in the aged rat penis: mechanism for age-associated erectile dysfunction. FASEB J. 2006; 20:536–8.
Article30). Wang H, Eto M, Steers WD, Somlyo AP, Somlyo AV. RhoA-mediated Ca2+ sensitization in erectile function. J Biol Chem. 2002; 277:30614–21.31). Sakamoto T, Repasky WT, Uchida K, Hirata A, Hirata F. Modulation of cell death pathways to apoptosis and necrosis of H2O2-treated rat thymocytes by lipocortin I. Biochem Biophys Res Commun. 1996; 220:643–7.
Article32). Parente L, Solito E. Annexin 1: more than an anti-phospholipase protein. Inflamm Res. 2004; 53:125–32.
Article33). Townsend DM, Tew KD. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene. 2003; 22:7369–75.
Article34). Bambang IF, Xu S, Zhou J, Salto-Tellez M, Sethi SK, Zhang D. Overexpression of endoplasmic reticulum protein 29 regulates mesenchymal-epithelial transition and suppresses xenograft tumor growth of invasive breast cancer cells. Lab Invest. 2009; 89:1229–42.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Proteomic Analysis of Penile Protein Alterations in a Rat Model of Cavernous Nerve Injury
- Penile erection evoked by autonomic nerve stimulation in rats
- Effect of Androgen Deprivation and Replacement on the Penis and Erectile Function in the Adult Rat
- Relaxin-2 Prevents Erectile Dysfunction by Cavernous Nerve, Endothelial and Histopathological Protection Effects in Rats with Bilateral Cavernous Nerve Injury
- Argonaute 2 restored erectile function and corpus cavernosum mitochondrial function by reducing apoptosis in a mouse model of cavernous nerve injury