Korean Circ J.
2012 Apr;42(4):239-245. 10.4070/kcj.2012.42.4.239.
Kruppel-Like Factor 2 Suppression by High Glucose as a Possible Mechanism of Diabetic Vasculopathy
- Affiliations
-
- 1Cardiovascular Laboratory, Clinical Research Institute, Seoul National University Hospital, Seoul, Korea. hylee612@snu.ac.kr
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2225016
- DOI: http://doi.org/10.4070/kcj.2012.42.4.239
Abstract
- BACKGROUND AND OBJECTIVES
Endothelial dysfunction is widely observed in diabetes mellitus, resulting in diabetic vascular complications. Kruppel-like factor 2 (KLF2) is implicated as being a key molecule that maintains endothelial function. We evaluated the expression of KLF2 in endothelial cells cultured in high glucose and investigated its functional implication in a diabetic animal model.
SUBJECTS AND METHODS
Human umbilical vein endothelial cells (HUVECs) were cultured in physiologically high glucose (35 mM) condition. The Otsuka Long Evans Tokushima Fatty (OLETF) strain of rat was used as an excellent model of obese type II diabetes, and their lean littermates are Long Evans Tokushima Otsuka (LETO) rats.
RESULTS
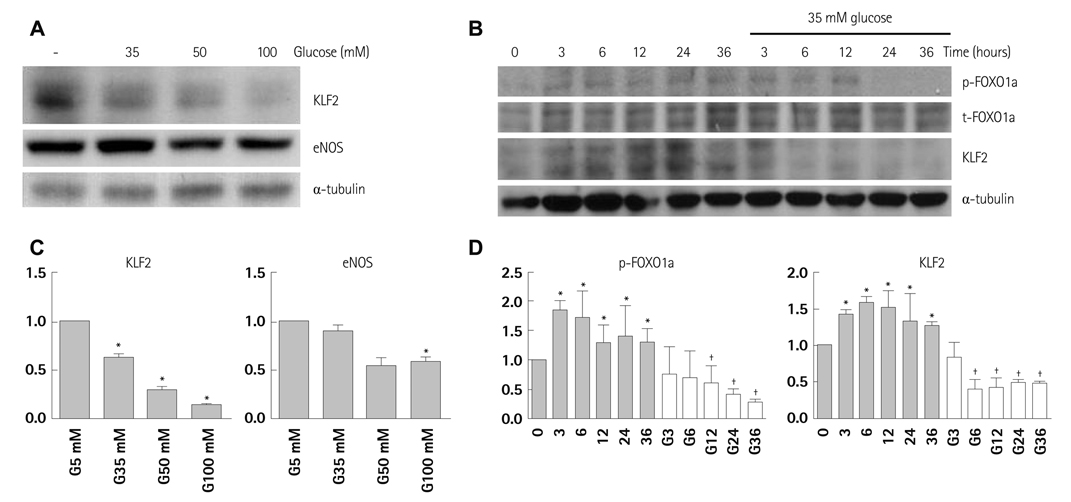
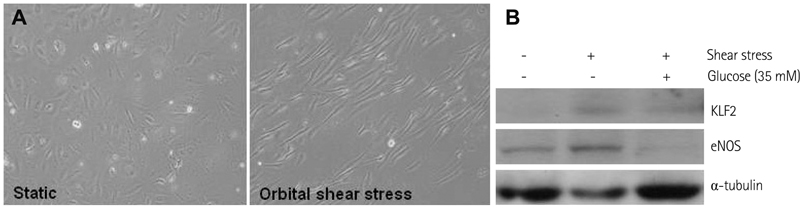
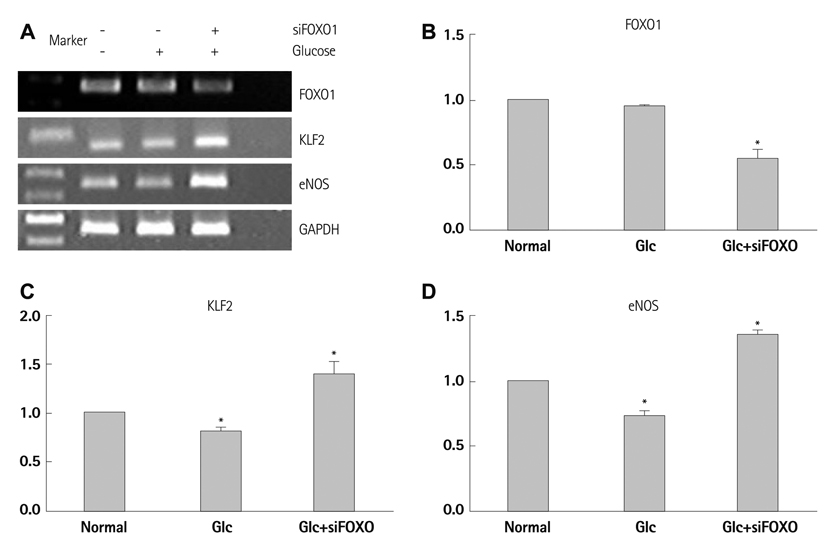
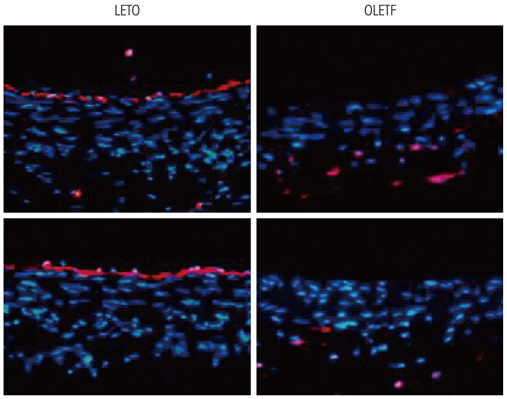
In HUVECs cultured in physiologically high glucose condition, FOXO1 was activated whereas KLF2 and endothelial nitric oxide synthase (eNOS) expression was near completely abolished, which was completely reversed by FOXO1 small interfering ribonucleic acid. In the vessels harvested from the OLETF rats, the animal model of type II diabetes, KLF2 and eNOS expression were found depleted. When vascular remodeling was induced in the left common carotid artery by reduction of blood flow with partial ligation of the distal branches, greater neointimal hypertrophy was observed in OLETF rats compared with the control LETO rats.
CONCLUSION
KLF2 suppression in endothelial cells by high glucose is a possible mechanism of diabetic endothelial dysfunction. The strategy of replenishing KLF2 may be effective for preventing diabetic vascular dysfunction.
MeSH Terms
Figure
Reference
-
1. Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998. 339:229–234.2. Hink U, Li H, Mollnau H, et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001. 88:E14–E22.3. De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000. 130:963–974.4. Johnstone MT, Creager SJ, Scales KM, Cusco JA, Lee BK, Creager MA. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation. 1993. 88:2510–2516.5. Balletshofer BM, Rittig K, Enderle MD, et al. Endothelial dysfunction is detectable in young normotensive first-degree relatives of subjects with type 2 diabetes in association with insulin resistance. Circulation. 2000. 101:1780–1784.6. Hattori Y, Kawasaki H, Abe K, Kanno M. Superoxide dismutase recovers altered endothelium-dependent relaxation in diabetic rat aorta. Am J Physiol. 1991. 261(4 Pt 2):H1086–H1094.7. Beckman JA, Goldfine AB, Gordon MB, Garrett LA, Creager MA. Inhibition of protein kinase Cbeta prevents impaired endothelium-dependent vasodilation caused by hyperglycemia in humans. Circ Res. 2002. 90:107–111.8. Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction: implications for the syndrome of insulin resistance. J Clin Invest. 1996. 97:2601–2610.9. Atkins GB, Jain MK. Role of Krüppel-like transcription factors in endothelial biology. Circ Res. 2007. 100:1686–1695.10. Samatar AA, Wang L, Mirza A, Koseoglu S, Liu S, Kumar CC. Transforming growth factor-beta 2 is a transcriptional target for Akt/protein kinase B via forkhead transcription factor. J Biol Chem. 2002. 277:28118–28126.11. Buteau J, Accili D. Regulation of pancreatic beta-cell function by the forkhead protein FoxO1. Diabetes Obes Metab. 2007. 9:Suppl 2. 140–146.12. Kamagate A, Qu S, Perdomo G, et al. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J Clin Invest. 2008. 118:2347–2364.13. Kim HS, Skurk C, Thomas SR, et al. Regulation of angiogenesis by glycogen synthase kinase-3beta. J Biol Chem. 2002. 277:41888–41896.14. Kanemoto N, Hishigaki H, Miyakita A, et al. Genetic dissection of "OLETF", a rat model for non-insulin-dependent diabetes mellitus. Mamm Genome. 1998. 9:419–425.15. Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol. 1997. 17:2238–2244.16. Korshunov VA, Berk BC. Flow-induced vascular remodeling in the mouse: a model for carotid intima-media thickening. Arterioscler Thromb Vasc Biol. 2003. 23:2185–2191.17. Wang N, Miao H, Li YS, et al. Shear stress regulation of Krüppel-like factor 2 expression is flow pattern-specific. Biochem Biophys Res Commun. 2006. 341:1244–1251.18. Parmar KM, Larman HB, Dai G, et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006. 116:49–58.19. Hurks R, Eisinger MJ, Goovaerts I, et al. Early endothelial dysfunction in young type 1 diabetics. Eur J Vasc Endovasc Surg. 2009. 37:611–615.20. Baris N, Akdeniz B, Uyar S, et al. Are complex coronary lesions more frequent in patients with diabetes mellitus? Can J Cardiol. 2006. 22:935–937.21. Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005. 293:2126–2130.22. Parmar KM, Nambudiri V, Dai G, Larman HB, Gimbrone MA Jr, García-Cardeña G. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J Biol Chem. 2005. 280:26714–26719.23. Rossi J, Rouleau L, Tardif JC, Leask RL. Effect of simvastatin on Kruppel-like factor2, endothelial nitric oxide synthase and thrombomodulin expression in endothelial cells under shear stress. Life Sci. 2010. 87:92–99.24. Arslan F, Pasterkamp G, de Kleijn DP. Unraveling pleiotropic effects of statins: bit by bit, a slow case with perspective. Circ Res. 2008. 103:334–336.25. Thum T, Bauersachs J. Sports or statins for atheroprotection? New insight from Kruppel-like factor 2. Cardiovasc Res. 2006. 72:193–195.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of Sarcoplasmic Reticulum Ca2+ Uptake by Pyruvate and Fatty Acid in H9c2 Cardiomyocytes: Implications for Diabetic Cardiomyopathy
- Effect of Antiplatelets in Diabetic Peripheral Vasculopathy: Comparison by Ankle-Brachial Index and Peak Wave Velocity
- Regulatory Effects of O-GlcNAcylation in Vascular Smooth Muscle Cells on Diabetic Vasculopathy
- The Role of the Kidney in Glucose Metabolism
- Effects of Troglitazone on the Expression of VEGF and TGF-beta in Cultured Rat Mesangial Cells