Korean Circ J.
2013 Sep;43(9):581-591. 10.4070/kcj.2013.43.9.581.
Mercury Promotes Catecholamines Which Potentiate Mercurial Autoimmunity and Vasodilation: Implications for Inositol 1,4,5-Triphosphate 3-Kinase C Susceptibility in Kawasaki Syndrome
- Affiliations
-
- 1Shawnee, KS, USA.
- 2Department of Pharmaceutical Sciences, Northeastern University, Boston, MA, USA.
- 3Division of Allergy, Immunology and Rheumatology, Department of Pediatrics, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan. erickuo48@yahoo.com.tw
- KMID: 2224813
- DOI: http://doi.org/10.4070/kcj.2013.43.9.581
Abstract
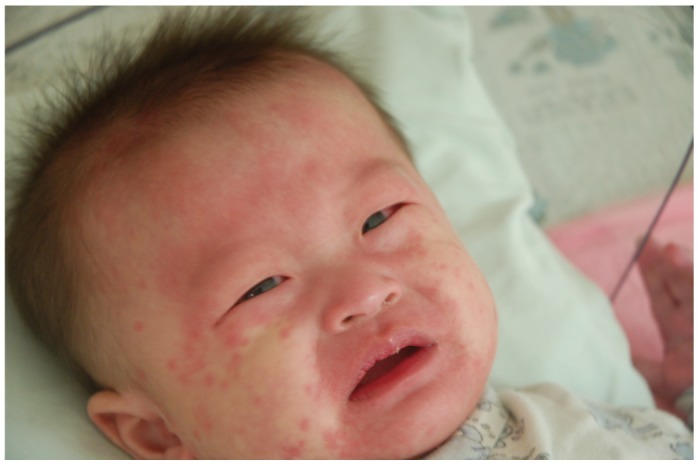
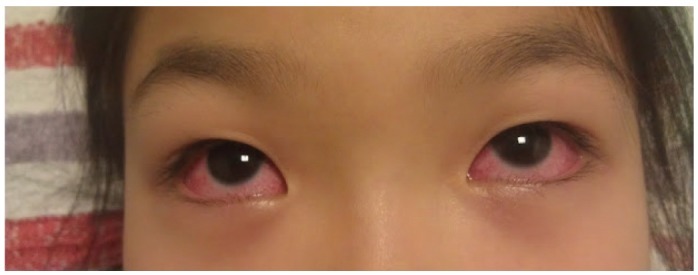
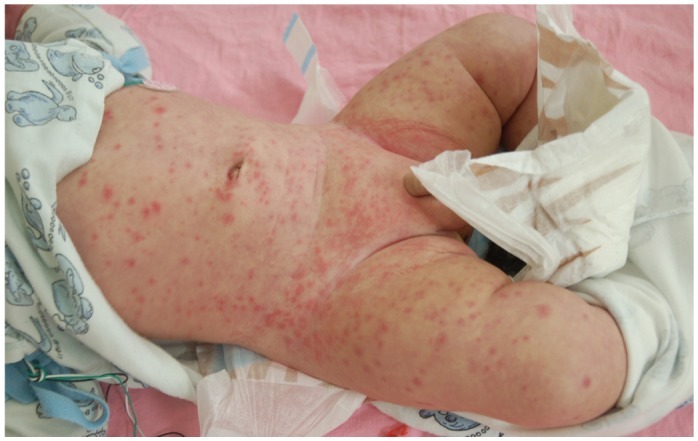
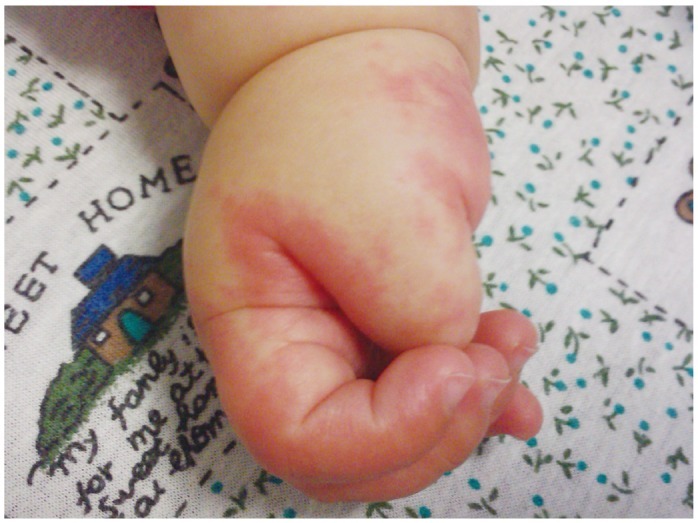
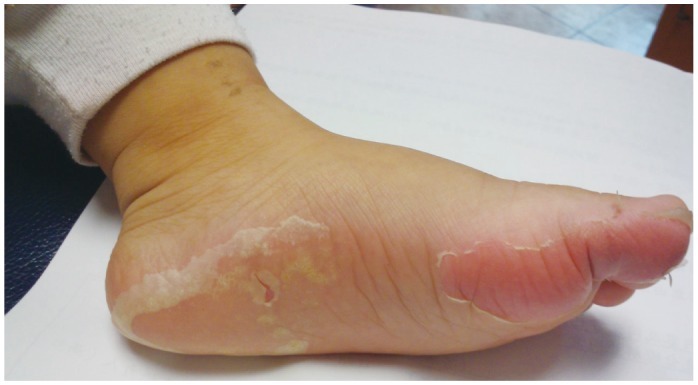
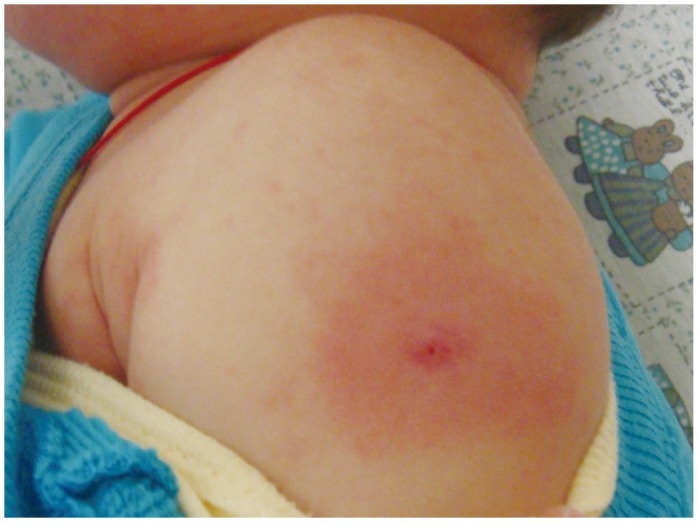
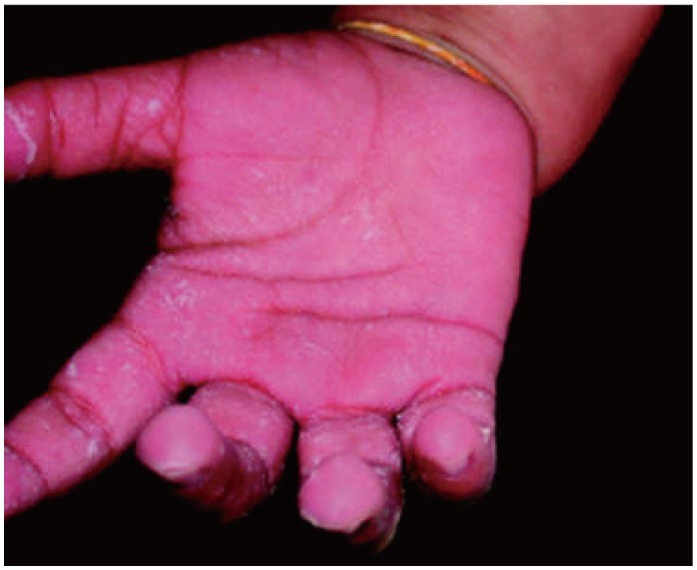
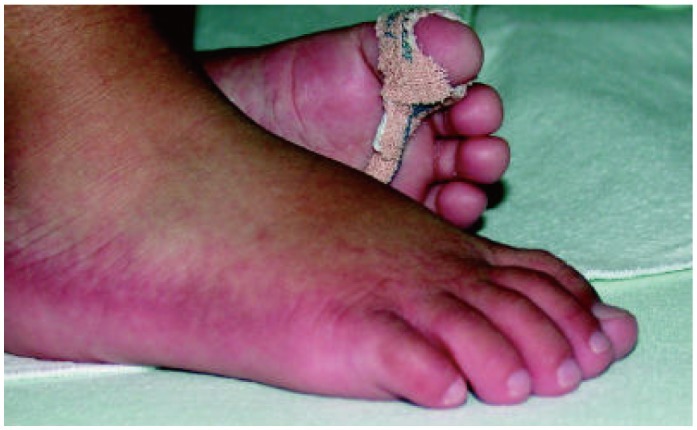
- Previously, we reviewed biological evidence that mercury could induce autoimmunity and coronary arterial wall relaxation as observed in Kawasaki syndrome (KS) through its effects on calcium signaling, and that inositol 1,4,5-triphosphate 3-kinase C (ITPKC) susceptibility in KS would predispose patients to mercury by increasing Ca2+ release. Hg2+ sensitizes inositol 1,4,5-triphosphate (IP3) receptors at low doses, which release Ca2+ from intracellular stores in the sarcoplasmic reticulum, resulting in delayed, repetitive calcium influx. ITPKC prevents IP3 from triggering IP3 receptors to release calcium by converting IP3 to inositol 1,3,4,5-tetrakisphosphate. Defective IP3 phosphorylation resulting from reduced genetic expressions of ITPKC in KS would promote IP3, which increases Ca2+ release. Hg2+ increases catecholamine levels through the inhibition of S-adenosylmethionine and subsequently catechol-O-methyltransferase (COMT), while a single nucleotide polymorphism of the COMT gene (rs769224) was recently found to be significantly associated with the development of coronary artery lesions in KS. Accumulation of norepinephrine or epinephrine would potentiate Hg2+-induced calcium influx by increasing IP3 production and increasing the permeability of cardiac sarcolemma to Ca2+. Norepinephrine and epinephrine also promote the secretion of atrial natriuretic peptide, a potent vasodilator that suppresses the release of vasoconstrictors. Elevated catecholamine levels can induce hypertension and tachycardia, while increased arterial pressure and a rapid heart rate would promote arterial vasodilation and subsequent fatal thromboses, particularly in tandem. Genetic risk factors may explain why only a susceptible subset of children develops KS although mercury exposure from methylmercury in fish or thimerosal in pediatric vaccines is nearly ubiquitous. During the infantile acrodynia epidemic, only 1 in 500 children developed acrodynia whereas mercury exposure was very common due to the use of teething powders. This hypothesis mirrors the leading theory for KS in which a widespread infection only induces KS in susceptible children. Acrodynia can mimic the clinical picture of KS, leading to its inclusion in the differential diagnosis for KS. Catecholamine levels are often elevated in acrodynia and may also play a role in KS. We conclude that KS may be the acute febrile form of acrodynia.
Keyword
MeSH Terms
-
Acrodynia
Arterial Pressure
Autoimmunity
Calcium
Calcium Signaling
Catechol O-Methyltransferase
Catecholamines
Child
Coronary Vessels
Diagnosis, Differential
Epinephrine
Heart Rate
Humans
Hydrazines
Hypertension
Inositol
Inositol 1,4,5-Trisphosphate
Inositol 1,4,5-Trisphosphate Receptors
Inositol Phosphates
Mucocutaneous Lymph Node Syndrome
Norepinephrine
Permeability
Phosphorylation
Polymorphism, Single Nucleotide
Powders
Relaxation
Risk Factors
S-Adenosylmethionine
Sarcolemma
Sarcoplasmic Reticulum
Tachycardia
Thimerosal
Thrombosis
Tooth
Tooth Eruption
Vaccines
Vasoconstrictor Agents
Vasodilation
Calcium
Catechol O-Methyltransferase
Catecholamines
Epinephrine
Hydrazines
Inositol
Inositol 1,4,5-Trisphosphate
Inositol 1,4,5-Trisphosphate Receptors
Inositol Phosphates
Norepinephrine
Powders
S-Adenosylmethionine
Thimerosal
Vaccines
Vasoconstrictor Agents
Figure
Reference
-
1. Adler R, Boxstein D, Schaff P, Kelly D. Metallic mercury vapor poisoning simulating mucocutaneous lymph node syndrome. J Pediatr. 1982; 101:967–968. PMID: 7143177.2. Mason WH, Takahashi M. Kawasaki syndrome. Clin Infect Dis. 1999; 28:169–185. quiz 186-7. PMID: 10064222.3. Nakamura Y, Yashiro M, Uehara R, et al. Epidemiologic features of Kawasaki disease in Japan: results of the 2009-2010 nationwide survey. J Epidemiol. 2012; 22:216–221. PMID: 22447211.4. Huang WC, Huang LM, Chang IS, et al. Epidemiologic features of Kawasaki disease in Taiwan, 2003-2006. Pediatrics. 2009; 123:e401–e405. PMID: 19237439.5. Park YW, Han JW, Hong YM, et al. Epidemiological features of Kawasaki disease in Korea, 2006-2008. Pediatr Int. 2011; 53:36–39. PMID: 20534021.6. Onouchi Y. Genetics of Kawasaki disease: what we know and don't know. Circ J. 2012; 76:1581–1586. PMID: 22789975.7. Khor CC, Davila S, Breunis WB, et al. Genome-wide association study identifies FCGR2A as a susceptibility locus for Kawasaki disease. Nat Genet. 2011; 43:1241–1246. PMID: 22081228.8. Kuo HC, Yang KD, Juo SH, et al. ITPKC single nucleotide polymorphism associated with the Kawasaki disease in a Taiwanese population. PLoS One. 2011; 6:e17370. PMID: 21533171.9. Lin MT, Wang JK, Yeh JI, et al. Clinical Implication of the C Allele of the ITPKC Gene SNP rs28493229 in Kawasaki Disease: Association With Disease Susceptibility and BCG Scar Reactivation. Pediatr Infect Dis J. 2011; 30:148–152. PMID: 20805785.10. Lou J, Xu S, Zou L, et al. A functional polymorphism, rs28493229, in ITPKC and risk of Kawasaki disease: an integrated meta-analysis. Mol Biol Rep. 2012; 39:11137–11144. PMID: 23065250.11. Onouchi Y, Gunji T, Burns JC, et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat Genet. 2008; 40:35–42. PMID: 18084290.12. Onouchi Y, Suzuki Y, Suzuki H, et al. ITPKC and CASP3 polymorphisms and risks for IVIG unresponsiveness and coronary artery lesion formation in Kawasaki disease. Pharmacogenomics J. 2013; 13:52–59. PMID: 21987091.13. Chi H, Huang FY, Chen MR, et al. ITPKC gene SNP rs28493229 and Kawasaki disease in Taiwanese children. Hum Mol Genet. 2010; 19:1147–1151. PMID: 20045869.14. Peng Q, Chen CH, Wu Q, et al. [Association study of a functional SNP rs28493229 of ITPKC gene and Kawasaki disease in a Chinese population]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2011; 28:644–648. PMID: 22161096.15. Peng Q, Chen C, Zhang Y, et al. Single-nucleotide polymorphism rs2290692 in the 3'UTR of ITPKC associated with susceptibility to Kawasaki disease in a Han Chinese population. Pediatr Cardiol. 2012; 33:1046–1053. PMID: 22361738.16. Tremoulet AH, Pancoast P, Franco A, et al. Calcineurin inhibitor treatment of intravenous immunoglobulin-resistant Kawasaki disease. J Pediatr. 2012; 161:506–512.e1. PMID: 22484354.17. Bultynck G, Szlufcik K, Kasri NN, et al. Thimerosal stimulates Ca2+ flux through inositol 1,4,5-trisphosphate receptor type 1, but not type 3, via modulation of an isoform-specific Ca2+-dependent intramolecular interaction. Biochem J. 2004; 381(Pt 1):87–96. PMID: 15015936.18. Edwards DH, Li Y, Griffith TM. Hydrogen peroxide potentiates the EDHF phenomenon by promoting endothelial Ca2+ mobilization. Arterioscler Thromb Vasc Biol. 2008; 28:1774–1781. PMID: 18669883.19. Goth SR, Chu RA, Gregg JP, Cherednichenko G, Pessah IN. Uncoupling of ATP-mediated calcium signaling and dysregulated interleukin-6 secretion in dendritic cells by nanomolar thimerosal. Environ Health Perspect. 2006; 114:1083–1091. PMID: 16835063.20. Joseph SK, Nakao SK, Sukumvanich S. Reactivity of free thiol groups in type-I inositol trisphosphate receptors. Biochem J. 2006; 393(Pt 2):575–582. PMID: 16173918.21. Yeter D, Deth R. ITPKC susceptibility in Kawasaki syndrome as a sensitizing factor for autoimmunity and coronary arterial wall relaxation induced by thimerosal's effects on calcium signaling via IP3. Autoimmun Rev. 2012; 11:903–908. PMID: 22498790.22. Crack P, Cocks T. Thimerosal blocks stimulated but not basal release of endothelium-derived relaxing factor (EDRF) in dog isolated coronary artery. Br J Pharmacol. 1992; 107:566–572. PMID: 1384915.23. Rosenblum WI, Nishimura H, Ellis EF, Nelson GH. The endothelium-dependent effects of thimerosal on mouse pial arterioles in vivo: evidence for control of microvascular events by EDRF as well as prostaglandins. J Cereb Blood Flow Metab. 1992; 12:703–706. PMID: 1618948.24. Bény JL. Thimerosal hyperpolarizes arterial smooth muscles in an endothelium-dependent manner. Eur J Pharmacol. 1990; 185:235–238. PMID: 2123797.25. Förstermann U, Goppelt-Strübe M, Frölich JC, Busse R. Thimerosal, an inhibitor of endothelial acyl-coenzyme A: lysolecithin acyltransferase, stimulates the production of a nonprostanoid endothelium-derived vascular relaxing factor. Adv Prostaglandin Thromboxane Leukot Res. 1987; 17B:1108–1111. PMID: 2960154.26. Förstermann U, Burgwitz K, Frölich JC. Thimerosal induces endothelium-dependent vascular smooth muscle relaxations by interacting with thiol groups. Relaxations are likely to be mediated by endothelium-derived relaxing factor (EDRF). Naunyn Schmiedebergs Arch Pharmacol. 1986; 334:501–507. PMID: 3102978.27. Beck C, Krafchik B, Traubici J, Jacobson S. Mercury intoxication: it still exists. Pediatr Dermatol. 2004; 21:254–259. PMID: 15165207.28. Brannan EH, Su S, Alverson BK. Elemental mercury poisoning presenting as hypertension in a young child. Pediatr Emerg Care. 2012; 28:812–814. PMID: 22863825.29. Michaeli-Yossef Y, Berkovitch M, Goldman M. Mercury intoxication in a 2-year-old girl: a diagnostic challenge for the physician. Pediatr Nephrol. 2007; 22:903–906. PMID: 17310361.30. Torres AD, Rai AN, Hardiek ML. Mercury intoxication and arterial hypertension: report of two patients and review of the literature. Pediatrics. 2000; 105:E34. PMID: 10699136.31. Lim YJ, Jung JW, Jung HJ, Park JE. Two Cases of Kawasaki Disease with Hidden Neuroblastoma. Indian J Pediatr. 2012; [Epub ahead of print].32. Ohta S, Narita T, Kato H, Taga T, Takeuchi Y. A patient with Kawasaki disease who developed acute urinary retention due to pelvic neuroblastoma. Eur J Pediatr. 2002; 161:631. PMID: 12532947.33. Lee HJ, Lee MS, Kim JS, et al. The relationship between catechol-O-methyltransferase gene polymorphism and coronary artery abnormality in Kawasaki disease. Korean J Pediatr. 2009; 52:87–92.34. Fukazawa R, Sonobe T, Hamamoto K, et al. Possible synergic effect of angiotensin-I converting enzyme gene insertion/deletion polymorphism and angiotensin-II type-1 receptor 1166A/C gene polymorphism on ischemic heart disease in patients with Kawasaki disease. Pediatr Res. 2004; 56:597–601. PMID: 15295089.35. Shim YH, Kim HS, Sohn S, Hong YM. Insertion/deletion polymorphism of angiotensin converting enzyme gene in Kawasaki disease. J Korean Med Sci. 2006; 21:208–211. PMID: 16614502.36. Takeuchi K, Yamamoto K, Kataoka S, et al. High incidence of angiotensin I converting enzyme genotype II in Kawasaki disease patients with coronary aneurysm. Eur J Pediatr. 1997; 156:266–268. PMID: 9128808.37. Wu SF, Chang JS, Peng CT, Shi YR, Tsai FJ. Polymorphism of angiotensin-1 converting enzyme gene and Kawasaki disease. Pediatr Cardiol. 2004; 25:529–533. PMID: 15164145.38. Falcini F, Cerinic MM, Ermini M, et al. Nerve growth factor circulating levels are increased in Kawasaki disease: correlation with disease activity and reduced angiotensin converting enzyme levels. J Rheumatol. 1996; 23:1798–1802. PMID: 8895162.39. Falcini F, Generini S, Pignone A, et al. Are Angiotensin Converting Enzyme and von Willebrand factor circulating levels useful surrogate parameters to monitor disease activity in Kawasaki disease? Endothelium. 1999; 6:209–215. PMID: 10365772.40. Matucci-Cerinic M, Jaffa A, Kahaleh B. Angiotensin converting enzyme: an in vivo and in vitro marker of endothelial injury. J Lab Clin Med. 1992; 120:428–433. PMID: 1325530.41. Inoue N, Takai S, Jin D, et al. Effect of angiotensin-converting enzyme inhibitor on matrix metalloproteinase-9 activity in patients with Kawasaki disease. Clin Chim Acta. 2010; 411:267–269. PMID: 19945447.42. Kudoh A, Kudoh E, Katagai H, Takazawa T. Norepinephrine-induced inositol 1,4,5-trisphosphate formation in atrial myocytes is regulated by extracellular calcium, protein kinase C, and calmodulin. Jpn Heart J. 2003; 44:547–556. PMID: 12906036.43. Fujiwara T, Fujiwara H, Takemura G, et al. Expression and distribution of atrial natriuretic polypeptide in the ventricles of children with myocarditis and/or myocardial infarction secondary to Kawasaki disease: immunohistochemical study. Am Heart J. 1990; 120:612–618. PMID: 2143884.44. Bang S, Yu JJ, Han MK, et al. Log-transformed plasma level of brain natriuretic peptide during the acute phase of Kawasaki disease is quantitatively associated with myocardial dysfunction. Korean J Pediatr. 2011; 54:340–344. PMID: 22087201.45. Cho SY, Kim Y, Cha SH, Suh JT, Han MY, Lee HJ. Adjuvant laboratory marker of Kawasaki disease; NT-pro-BNP or hs-CRP? Ann Clin Lab Sci. 2011; 41:360–363. PMID: 22166506.46. Dahdah N, Siles A, Fournier A, et al. Natriuretic peptide as an adjunctive diagnostic test in the acute phase of Kawasaki disease. Pediatr Cardiol. 2009; 30:810–817. PMID: 19365652.47. Kawamura T, Wago M, Kawaguchi H, Tahara M, Yuge M. Plasma brain natriuretic peptide concentrations in patients with Kawasaki disease. Pediatr Int. 2000; 42:241–248. PMID: 10881579.48. Kishimoto S, Suda K, Teramachi Y, et al. Increased plasma type B natriuretic peptide in the acute phase of Kawasaki disease. Pediatr Int. 2011; 53:736–741. PMID: 21410593.49. Kurotobi S, Kawakami N, Shimizu K, et al. Brain natriuretic peptide as a hormonal marker of ventricular diastolic dysfunction in children with Kawasaki disease. Pediatr Cardiol. 2005; 26:425–430. PMID: 15633045.50. No SJ, Kim DO, Choi KM, Eun LY. Do predictors of incomplete Kawasaki disease exist for infants? Pediatr Cardiol. 2013; 34:286–290. PMID: 23001516.51. Sun YP, Wang WD, Zheng XC, Wang JJ, Ma SC, Xu YJ. [Levels of serum brain natriuretic peptide and the correlation to heart function in children with Kawasaki disease]. Zhongguo Dang Dai Er Ke Za Zhi. 2010; 12:169–171. PMID: 20350422.52. Takeuchi D, Saji T, Takatsuki S, Fujiwara M. Abnormal tissue doppler images are associated with elevated plasma brain natriuretic peptide and increased oxidative stress in acute Kawasaki disease. Circ J. 2007; 71:357–362. PMID: 17322635.53. Zhang QY, Du JB, Chen YH, Li WZ. [Change in plasma N-terminal probrain natriuretic peptide in children with Kawasaki disease and its value in clinical practice]. Zhonghua Er Ke Za Zhi. 2006; 44:886–890. PMID: 17254452.54. Mori J, Miura M, Shiro H, Fujioka K, Kohri T, Hasegawa T. Syndrome of inappropriate anti-diuretic hormone in Kawasaki disease. Pediatr Int. 2011; 53:354–357. PMID: 21029251.55. Watanabe T, Abe Y, Sato S, Uehara Y, Ikeno K, Abe T. Hyponatremia in Kawasaki disease. Pediatr Nephrol. 2006; 21:778–781. PMID: 16565868.56. Nakamura Y, Yashiro M, Uehara R, et al. Use of laboratory data to identify risk factors of giant coronary aneurysms due to Kawasaki disease. Pediatr Int. 2004; 46:33–38. PMID: 15043662.57. Lim GW, Lee M, Kim HS, Hong YM, Sohn S. Hyponatremia and syndrome of inappropriate antidiuretic hormone secretion in kawasaki disease. Korean Circ J. 2010; 40:507–513. PMID: 21088754.58. Ohta M, Ito S. [Hyponatremia and inflammation]. Rinsho Byori. 1999; 47:408–416. PMID: 10375961.59. Cheek DB, Hetzel BS, Hine DC. Evidence of adrenal cortical function in pink disease. Med J Aust. 1951; 2:6–8. PMID: 14862576.60. Cheek DB. Pink disease; infantile acrodynia; a physiological approach; an evaluation of adrenal function and the importance of water and electrolyte metabolism. J Pediatr. 1953; 42:239–260. PMID: 13023525.61. Hisatome I, Kurata Y, Sasaki N, et al. Block of sodium channels by divalent mercury: role of specific cysteinyl residues in the P-loop region. Biophys J. 2000; 79:1336–1345. PMID: 10968996.62. Kuo HC, Yu HR, Juo SH, et al. CASP3 gene single-nucleotide polymorphism (rs72689236) and Kawasaki disease in Taiwanese children. J Hum Genet. 2011; 56:161–165. PMID: 21160486.63. Onouchi Y, Ozaki K, Buns JC, et al. Common variants in CASP3 confer susceptibility to Kawasaki disease. Hum Mol Genet. 2010; 19:2898–2906. PMID: 20423928.64. Huang YC, Lin YJ, Chang JS, et al. Single nucleotide polymorphism rs2229634 in the ITPR3 gene is associated with the risk of developing coronary artery aneurysm in children with Kawasaki disease. Int J Immunogenet. 2010; 37:439–443. PMID: 20618519.65. Kuo HC, Lin YJ, Juo SH, et al. Lack of association between ORAI1/CRACM1 gene polymorphisms and Kawasaki disease in the Taiwanese children. J Clin Immunol. 2011; 31:650–655. PMID: 21487896.66. Braun A, Varga-Szabo D, Kleinschnitz C, et al. Orai1 (CRACM1) is the platelet SOC channel and essential for pathological thrombus formation. Blood. 2009; 113:2056–2063. PMID: 18832659.67. McCarl CA, Khalil S, Ma J, et al. Store-operated Ca2+ entry through ORAI1 is critical for T cell-mediated autoimmunity and allograft rejection. J Immunol. 2010; 185:5845–5858. PMID: 20956344.68. Kawasaki T, Ueyama T, Lange I, Feske S, Saito N. Protein kinase C-induced phosphorylation of Orai1 regulates the intracellular Ca2+ level via the store-operated Ca2+ channel. J Biol Chem. 2010; 285:25720–25730. PMID: 20534587.69. Caballero Mora FJ, Alvarez-Coca González J, Alonso Martín B, Martínez Pérez J. [Is the determination of procalcitonin useful in Kawasaki disease?]. An Pediatr (Barc). 2009; 71:371–372. PMID: 19726253.70. Okada Y, Minakami H, Tomomasa T, et al. Serum procalcitonin concentration in patients with Kawasaki disease. J Infect. 2004; 48:199–205. PMID: 14720497.71. Yoshikawa H, Nomura Y, Masuda K, et al. Serum procalcitonin value is useful for predicting severity of Kawasaki disease. Pediatr Infect Dis J. 2012; 31:523–525. PMID: 22189525.72. Catalano-Pons C, Andre MC, Chalumeau M, Guerin S, Gendrel D. Lack of value of procalcitonin for prediction of coronary aneurysms in Kawasaki disease. Pediatr Infect Dis J. 2007; 26:179–180. PMID: 17259884.73. Chakrabartty S, Apong S. Procalcitonin estimation in Kawasaki disease. Indian Pediatr. 2009; 46:648. PMID: 19638671.74. Sokolow S, Luu SH, Headley AJ, et al. High levels of synaptosomal Na(+)-Ca(2+) exchangers (NCX1, NCX2, NCX3) co-localized with amyloid-beta in human cerebral cortex affected by Alzheimer's disease. Cell Calcium. 2011; 49:208–216. PMID: 21382638.75. Staiano RI, Granata F, Secondo A, et al. Human macrophages and monocytes express functional Na(+)/Ca (2+) exchangers 1 and 3. Adv Exp Med Biol. 2013; 961:317–326. PMID: 23224891.76. Dixit M, Kim S, Matthews GF, et al. Structural arrangement of the intracellular Ca2+ binding domains of the cardiac Na+/Ca2+ exchanger (NCX1.1): effects of Ca2+ binding. J Biol Chem. 2013; 288:4194–4207. PMID: 23233681.77. Denny MF, Atchison WD. Mercurial-induced alterations in neuronal divalent cation homeostasis. Neurotoxicology. 1996; 17:47–61. PMID: 8784818.78. Hare MF, Atchison WD. Methylmercury mobilizes Ca++ from intracellular stores sensitive to inositol 1,4,5-trisphosphate in NG108-15 cells. J Pharmacol Exp Ther. 1995; 272:1016–1023. PMID: 7891311.79. Kawasaki T. Letter to the editor. Pediatrics. 1975; 56:336–337.80. Orlowski JP, Mercer RD. Urine mercury levels in Kawasaki disease. Pediatrics. 1980; 66:633–636. PMID: 7432853.81. Aschner M, Aschner JL. Mucocutaneous lymph node syndrome: is there a relationship to mercury exposure? Am J Dis Child. 1989; 143:1133–1134. PMID: 2801647.82. Mutter J. Is dental amalgam safe for humans? The opinion of the scientific committee of the European Commission. J Occup Med Toxicol. 2011; 6:2. PMID: 21232090.83. Deth RC. Congressional testimony before the U.S. House of Representatives. Subcommittee on Human Rights and Wellness;2004.84. United Nations Environment Program. Global Mercury Assessment Report. Geneva: United Nations;2002.85. Stoiber T, Bonacker D, Böhm KJ, et al. Disturbed microtubule function and induction of micronuclei by chelate complexes of mercury(II). Mutat Res. 2004; 563:97–106. PMID: 15364276.86. Thier R, Bonacker D, Stoiber T, et al. Interaction of metal salts with cytoskeletal motor protein systems. Toxicol Lett. 2003; 140-141:75–81. PMID: 12676453.87. Martindale W. The extra pharmacopoeia, vol 2. Pharmaceutical Society of Great Britain. London: Pharmaceutical Press;1982.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study on the Protective Effects of Glutathione on Cytotoxicity of Mercury and Cadmium
- A Study of Protective Effect of Selenium Against Cytotoxicity of Methylmercury Chloride
- The distribution of inositol 1,4,5-trisphosphate 3-kinase in rat cerebellum
- Purification of Inositol Triphosphate Kinase from Bovine Brain
- A Study of Cytotoxical Mechanism of Mercurial Compounds in RAW264.7 Cell Line