J Korean Rheum Assoc.
2009 Jun;16(2):123-132. 10.4078/jkra.2009.16.2.123.
Upregulation of Macrophage Migration Inhibitory Factor (MIF) Production by Engagement of Toll-like Receptor 3 (TLR3) on Fibroblast-like Synoviocyte (FLS) from Patients with Rheumatoid Arthritis
- Affiliations
-
- 1The Rheumatism Research Center, Catholic Research Institute of Medical Science, The Catholic University of Korea, Seoul, Korea.
- 2Division of Rheumatology, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. rapark@catholic.ac.kr
- KMID: 2202128
- DOI: http://doi.org/10.4078/jkra.2009.16.2.123
Abstract
OBJECTIVE
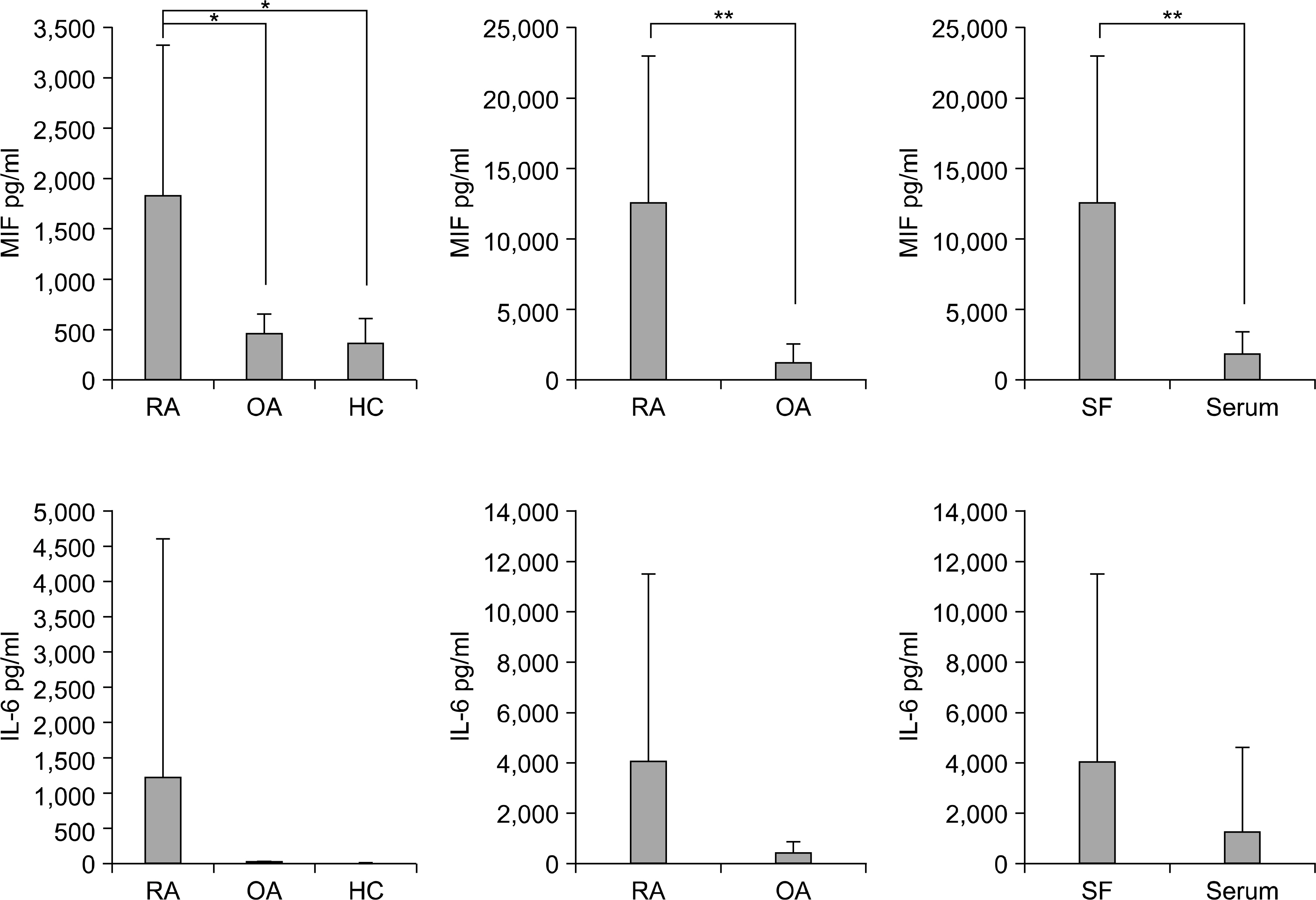
Rheumatoid arthritis (RA) is a chronic autoimmune disease. Macrophage migration inhibitory factor (MIF) has been shown to be an important pro-inflammatory cytokine in RA. The aim of this study was to determine if the engagement of toll-like receptor 3 (TLR3) induces the production of MIF in the fibroblast-like synoviocytes (FLS) of patients with RA.
METHODS
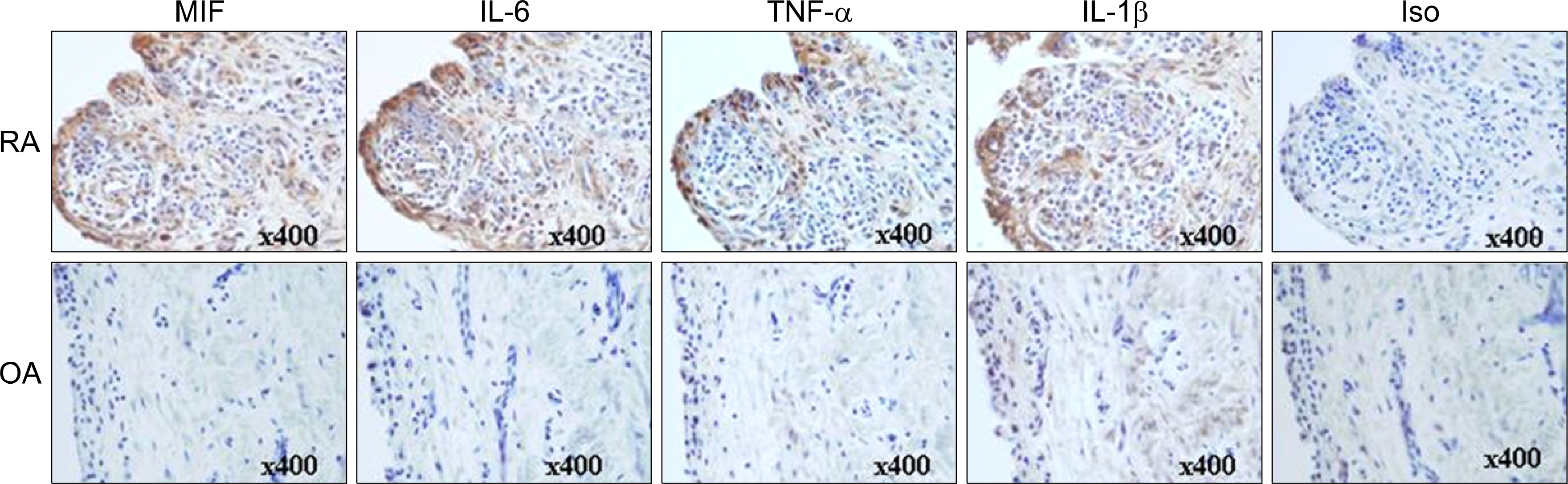
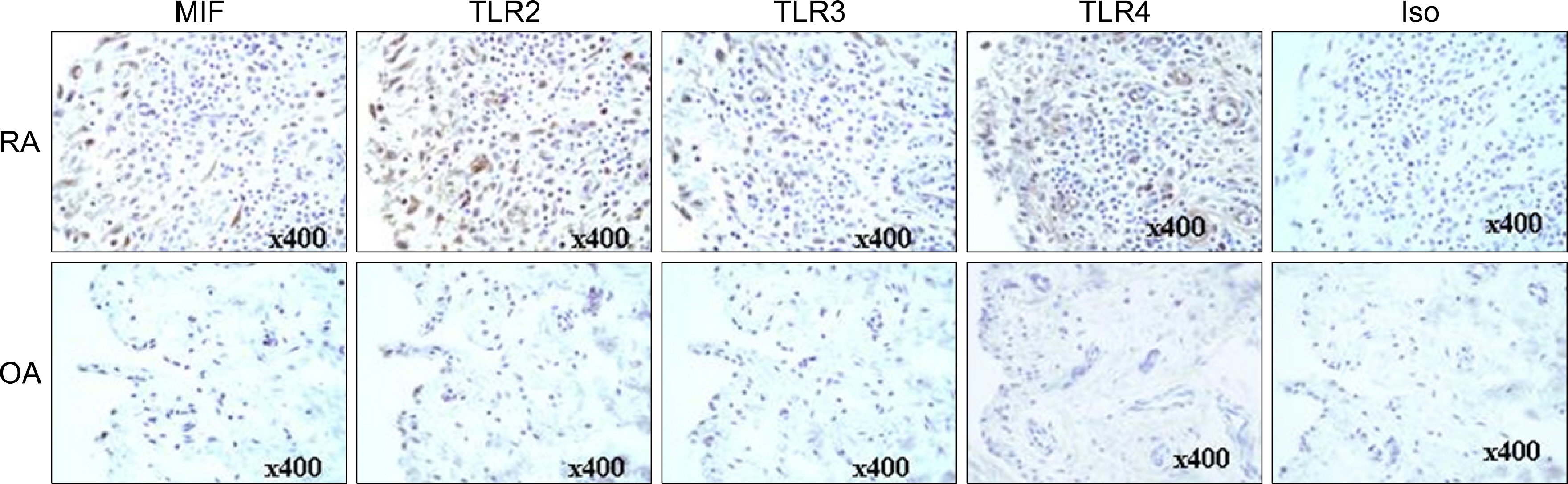
The expression of inflammatory cytokines (e.g. MIF, IL-6, IL-1beta and TNFalpha) and toll-like receptors (e.g. TLR2, TLR3 and TLR4) in the synovial tissue were quantified by immunohistochemistry. FLS were isolated from the synovial tissues of patients with RA and stimulated with TLR-3 ligand polyI:C, in the presence of a neutralizing antibody against IL-6. The concentrations of MIF and IL-6 in the culture supernatants from the FLS were measured using sandwich ELISA.
RESULTS
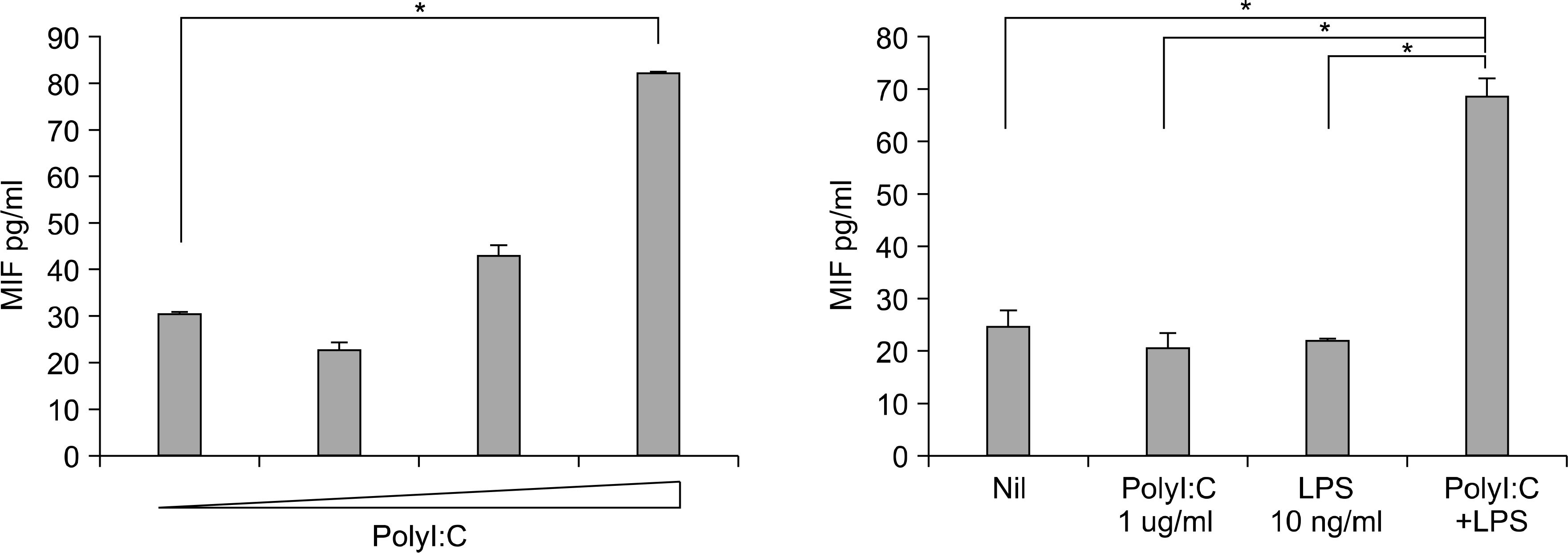
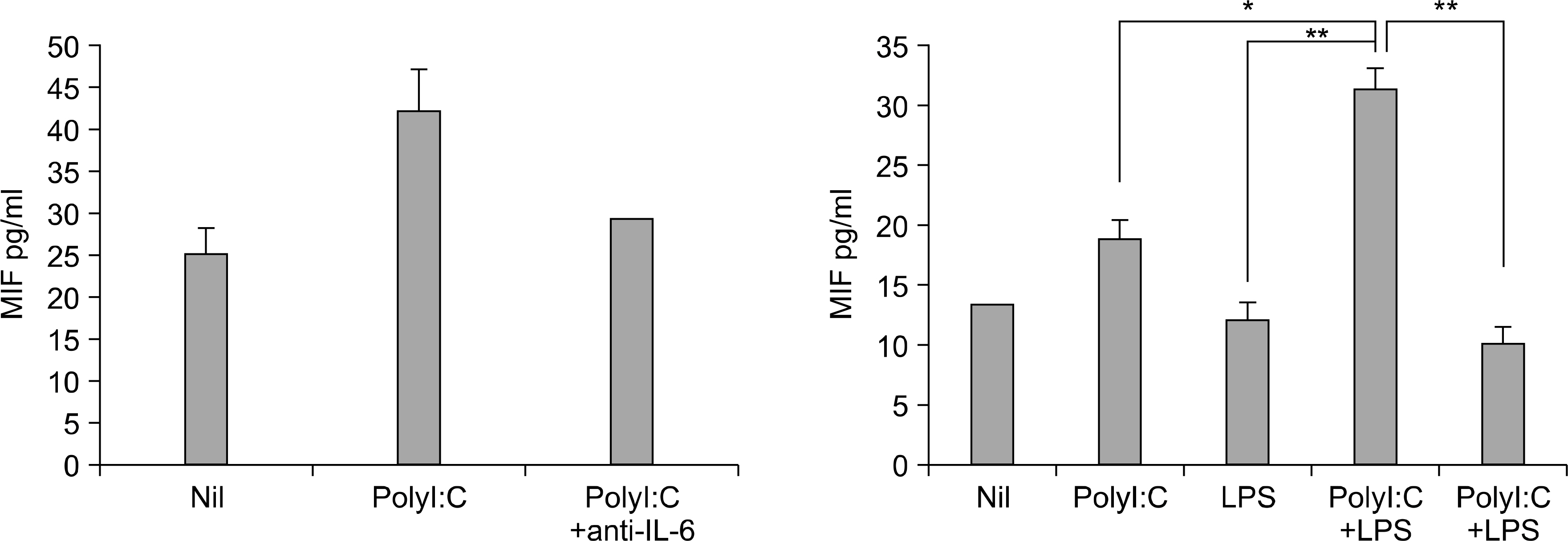
The engagement of TLR3 with PolyI:C increased the production of MIF in FLS. The stimulatory effect of these TLR ligands showed a dose-dependent trend. The combination of TLR3 and TLR4 synergistically increased the level of MIF and IL-6 production. The addition of neutralizing antibodies against IL-6 abrogated the stimulatory effect of the ligands of TLR3 and TLR4 on the production of MIF.
CONCLUSION
These results show that TLR3 engagement stimulates the production of MIF and IL-6. Therefore, the TLRs help perpetuate of RA pathogenesis through production of MIF from the FLS in patients with RA, and might provide a new therapeutic approach for the treatment of rheumatoid arthritis.
Keyword
MeSH Terms
-
Antibodies, Neutralizing
Arthritis, Rheumatoid
Autoimmune Diseases
Cytokines
Enzyme-Linked Immunosorbent Assay
Humans
Immunohistochemistry
Interleukin-6
Ligands
Macrophages
Toll-Like Receptor 3
Toll-Like Receptors
Up-Regulation
Antibodies, Neutralizing
Cytokines
Interleukin-6
Ligands
Toll-Like Receptor 3
Toll-Like Receptors
Figure
Reference
-
References
1. Kumar A, Zhang J, Yu FS. Toll-like receptor 3 agonist poly(I:C)-induced antiviral response in human corneal epithelial cells. Immunology. 2006; 117:11–21.
Article2. Takahashi N, Yamada T, Narita N, Fujieda S. Double-stranded RNA induces production of RANTES and IL-8 by human nasal fibroblasts. Clin Immunol. 2006; 118:51–8.
Article3. Proost P, Vynckier AK, Mahieu F, Put W, Grillet B, Struyf S, et al. Microbial Toll-like receptor ligands differentially regulate CXCL10/IP-10 expression in fibroblasts and mononuclear leukocytes in synergy with IFN-gamma and provide a mechanism for enhanced synovial chemokine levels in septic arthritis. Eur J lmmunol. 2003; 33:3146–53.4. Roelofs MF, Joosten LA, Abdollahi-Roodsaz S, van Lieshout AW, Sprong T, van den Hoogen FH, et al. The expression of toll-like receptors 3 and 7 in rheumatoid arthritis synovium is increased and costimulation of toll-like receptors 3, 4, and 7/8 results in synergistic cytokine production by dendritic cells. Arthritis Rheum. 2005; 52:2313–22.
Article5. Brentano F, Schorr O, Gay RE, Gay S, Kyburz D. RNA released from necrotic synovial fluid cells activates rheumatoid arthritis synovial fibroblasts via Toll-like receptor 3. Arthritis Rhwum. 2005; 52:2656–65.
Article6. Muller-Ladner U, Pap T. Pathogenesis of RA: more than just immune cells. Z Rheumatol. 2005; 64:396–401.7. Nishimoto N. Cytokine signal regulation and autoimmune disorders. Autoimmunity. 2005; 38:359–67.
Article8. Smolen JS, Steiner G. Therapeutic strategies for rheumatoid arthritis. Nature Rev Drug Discov. 2003; 2:473–88.
Article9. Nathan CF, Remold HG, David JR. Characterization of a lymphocyte factor which alters macrophage functions. J Exp Med. 1973; 137:275–90.
Article10. Burger-Kentischer A, Göbel H, Kleemann R, Zernecke A, Bucala R, Leng L, et al. Reduction of the aortic inflammatory response in spontaneous atherosclerosis by blockade of macrophage migration inhibitory factor (MIF). Atherosclerosis. 2006; 184:28–38.
Article11. Leech M, Metz C, Hall P, Hutchinson P, Gianis K, Smith M, et al. Macrophage migration inhibitory factor in rheumatoid arthritis: evidence of proinflammatory function and regulation by glucocorticoids. Arthritis Rheum. 1999; 42:1601–8.
Article12. Morand EF, Bucala R, Leech M. Macrophage migration inhibitory factor: an emerging therapeutic target in rheumatoid arthritis. Arthritis Rheum. 2003; 48:291–9.
Article13. Donnelly SC, Haslett C, Reid PT, Grant IS, Wallace WA, Metz CN, et al. Regulatory role for macrophage migration inhibitory factor in acute respiratory distress syndrome. Nat Med. 1997; 3:320–3.
Article14. Mizue Y, Ghani S, Leng L, McDonald C, Kong P, Baugh J, et al. Role for macrophage migration inhibitory factor in asthma. Proc Natl Acad Sci U S A. 2005; 102:14410–5.
Article15. Magalhães ES, Mourao-Sa DS, Vieira-de-Abreu A, Figueiredo RT, Pires AL, Farias-Filho FA, et al. Macrophage migration inhibitory factor is essential for allergic asthma but not for Th2 differentiation. Eur J Immunol. 2007; 37:1097–106.
Article16. Rossi AG, Haslett C, Hirani N, Greening AP, Rahman I, Metz CN, et al. Human circulating eosinophils secrete macrophage migration inhibitory factor (MIF). Potential role in asthma. J Clin Invest. 1998; 101:2869–74.
Article17. Bernhagen J, Calandra T, Mitchell RA, Martin SB, Tracey KJ, Voelter W, et al. MIF is a pituitaryderived cytokine that potentiates lethal endotoxaemia. Nature. 1993; 365:756–9.
Article18. Calandra T, Bernhagen J, Mitchell RA, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994; 179:1895–902.
Article19. Santos LL, Lacey D, Yang Y, Leech M, Morand EF. Activation of synovial cell p38 MAP kinase by macrophage migration inhibitory factor. J Rheumatol. 2004; 31:1038–43.20. Onodera S, Nishihira J, Koyama Y, Majima T, Aoki Y, Ichiyama H, et al. Macrophage migration inhibitory factor upregulates the expression of interleukin-8 messenger RNA in synovial fibroblasts of rheumatoid arthritis patients: common transcriptional regulatory mechanism between interleukin-8 and interleukin-1beta. Arthritis Rheum. 2004; 50:1437–47.21. Cunha FQ, Weiser WY, David JR, Moss DW, Mon-cada S, Liew FY. Recombinant migration inhibitory factor induces nitric oxide synthase in murine macrophages. J Immunol. 1993; 150:1908–12.22. Sampey AV, Hall PH, Mitchell RA, Metz CN, Morand EF. Regulation of synoviocyte phospholipase A2 and cyclooxygenase 2 by macrophage migration inhibitory factor. Arthritis Rheum. 2001; 44:1273–80.
Article23. Leech M, Metz C, Hall P, Hutchinson P, Gianis K, Smith M, et al. Macrophage migration inhibitory factor in rheumatoid arthritis: evidence of proinflammatory function and regulation by glucocorticoids. Arthritis Rheum. 1999; 42:1601–8.
Article24. Kim HR, Park MK, Cho ML, Yoon CH, Lee SH, Park SH, et al. Macrophage migration inhibitory factor upregulates angiogenic factors and correlates with clinical measures in rheumatoid arthritis. J Rheumatol. 2007; 34:927–36.25. Chesney J, Metz C, Bacher M, Peng T, Meinhardt A, Bucala R. An essential role for macrophage migration inhibitory factor (MIF) in angiogenesis and the growth of a murine lymphoma. Molecular Medicine. 1999; 5:181–91.
Article26. Amin MA, Volpert OV, Woods JM, Kumar P, Harlow LA, Koch AE. Migration inhibitory factor mediates angiogenesis via mitogen-activated protein kinase and phosphatidylinositol kinase. Circ Res. 2003; 93:321–9.
Article27. Roger T, David J, Glauser MP, Calandra T. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature. 2001; 414:920–4.
Article28. Bozza M, Satoskar AR, Lin G, Lu B, Humbles AA, Gerard C, et al. Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J Exp Med. 1999; 189:341–6.
Article29. Kyburz D, Rethage J, Seibl R, Lauener R, Gay RE, Carson DA, et al. Bacterial peptidoglycans but not CpG oligodeoxynucleotides activate synovial fibroblasts by Toll-like receptor signaling. Arthritis Rheum. 2003; 48:642–50.
Article30. Ospelt C, Brentano F, Rengel Y, Stanczyk J, Kolling C, Tak PP, et al. Overexpression of toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis: toll-like receptor expression in early and longstanding arthritis. Arthritis Rheum. 2008; 58:3684–92.
Article31. Onodera S, Tanji H, Suzuki K, Kaneda K, Mizue Y, Sagawa A, et al. High expression of macrophage migration inhibitory factor in the synovial tissues of rheumatoid joints. Cytokine. 1999; 11:163–7.
Article32. Nishihira J, Koyama Y, Mizue Y. Identification of macrophage migration inhibitory factor (MIF) in human vascular endothelial cells and its induction by lipopolysaccharide. Cytokine. 1998; 10:199–205.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Macrophage Migration Inhibitory Factor (MIF) Induced Stromal Cell-derived Factor 1 (SDF-1) Production Via Nuclear Factor KappaB (NF-kappaB) Signaling in Rheumatoid Arthritis Fibroblast Like Synoviocytes (RA-FLS)
- Macrophage migration inhibitory factor: a potential therapeutic target for rheumatoid arthritis
- Engagement of Toll-Like Receptor 3 Induces Vascular Endothelial Growth Factor and Interleukin-8 in Human Rheumatoid Synovial Fibroblasts
- Association between Macrophage Migration Inhibitory Factor Gene Polymorphism and Rheumatoid Arthritis
- Induction of Macrophage Migration Inhibitory Factor in ConA-Stimulated Rheumatoid Arthritis Synovial Fibroblasts through the P38 MAP Kinase-Dependent Signaling Pathway