J Korean Soc Endocrinol.
2006 Jun;21(3):192-203. 10.3803/jkes.2006.21.3.192.
Retrovirus Mediated Gene Transfer of RANK-Fc Ameliorates Bone Loss in a Mouse Ovariectomy Model of Osteoporosis
- Affiliations
-
- 1Department of Internal Medicine, Dankook University College of Medicine, Korea.
- 2Department of Internal Medicine, Seoul National University College of Medicine, Korea.
- KMID: 2200731
- DOI: http://doi.org/10.3803/jkes.2006.21.3.192
Abstract
-
BACKGROUND: Interactions between the receptor activator of the NF-kappaB ligand (RANKL) and its receptor, RANK, are important in the terminal differentiation and activation of osteoclasts. In the current investigation, we examine the feasibility of using genetically modified mesenchymal stem cells (MSCs), C3H10T1/2 cells as a platform for the sustained systemic delivery of therapeutic proteins into the circulation in an osteoporosis model, and investigate retroviral-mediated gene therapy of RANK-Fc as a means of ameliorating ovariectomy (OVX)-induced bone resorption.
METHODS
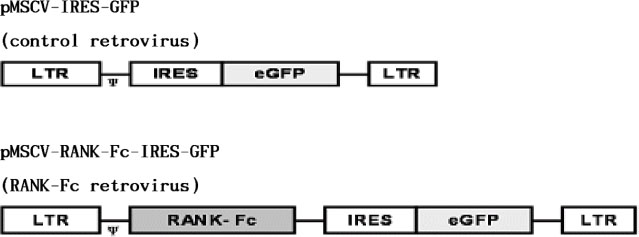
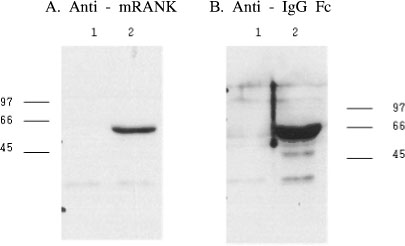
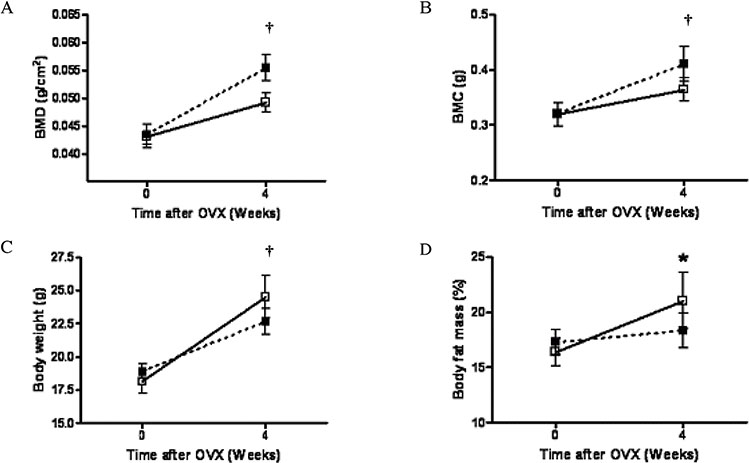
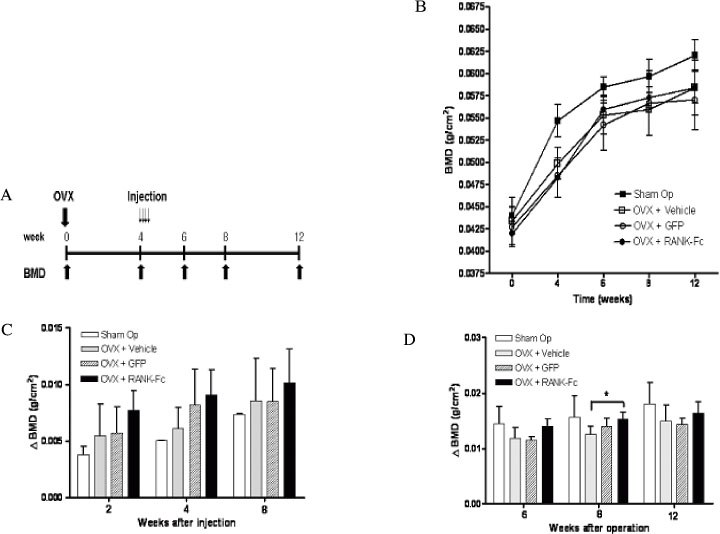
C3H10T1/2 cells were transduced with a MSCV-based retroviral vector containing cDNA of a fusion protein combining the extracellular domain of murine RANK with the human immunoglobulin constant domain (MSCV-RANK-Fc-eGFP). Young adult female mice were subjected to OVX or sham surgery, followed by treatment with transduced cells or PBS 4 weeks later. The expression of RANK-Fc by these cells was assessed, both in vitro and in vivo. Total bone mineral density (BMD) was measured and GFP expression was examined.
RESULTS
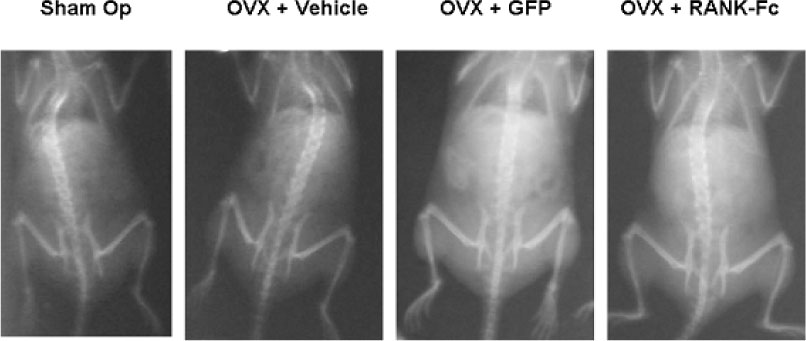
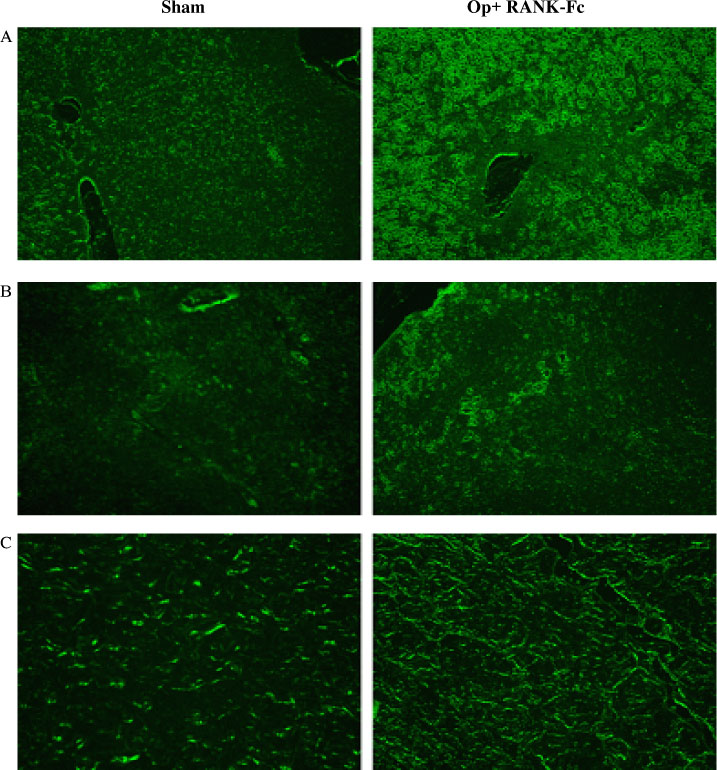
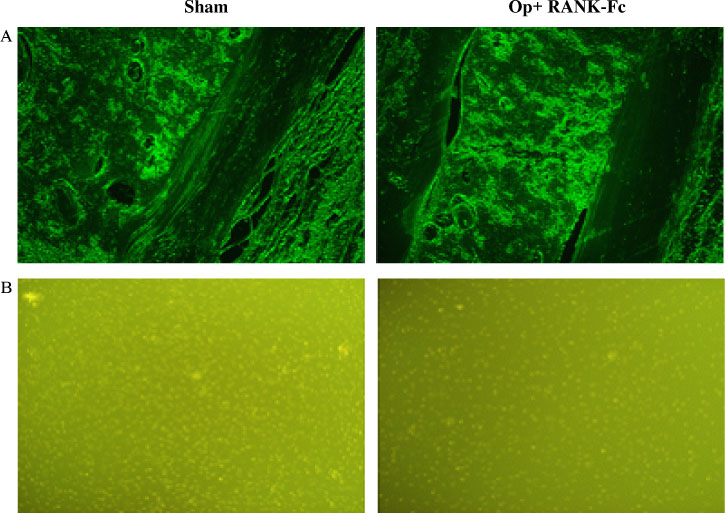
Transduced cells produced biologically active RANK-Fc in vitro and in vivo. Mice that were subjected to OVX followed by treatment with cells transduced with MSCV-RANK-Fc-eGFP 4 weeks later contained no significant but higher total BMD than either the control vector or PBS-treated mice after 8 weeks. Higher GFP expression was attained in the liver, spleen, and intra-abdominal fat of mice treated with MSCV-RANK-Fc-eGFP.
CONCLUSION
The data collectively indicate that C3H10T1/2 cells are effectively transduced with a MSCV-based retrovirus, and are capable of secreting biologically active RANK-Fc in vitro and in vivo. Moreover, gene therapy facilitating the sustained delivery of RANK-Fc may be an effective method to reverse OVX-induced osteoporosis.
MeSH Terms
Figure
Reference
-
1. The NIH consensus development panel on osteoporosis prevention, diagnosis, and therapy. JAMA. 2001. 285:785–795.2. Lopez FJ. New approaches to the treatment of osteoporosis. Curr Opin Chem Biol. 2000. 4:383–393.3. Fleisch H. Bisphosphonates: mechanisms of action. Endocr Rev. 1998. 19:80–100.4. Morley P, Whitfield JF, Willick GE. Parathyroid hormone; an anabolic treatment for osteoporosis. Curr Pharm Des. 2001. 7:671–689.5. Takahashi N, Udagawa N, Suda T. A new member of tumor necrosis factor ligand family, ODF/OPGL/TRANCE/RANKL, regulates osteoclast differentiation and function. Biochem Biophys Res Commun. 1999. 256:449–455.6. Hofbauer LC, Heufelder AE. Role of receptor activator of nuclear factor-κB ligand and osteoprotegerin in bone cell biology. J Mol Med. 2001. 79:243–253.7. Simonet WS, Lacey DL, Dunstan CR. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell. 1997. 89:309–319.8. Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998. 12:1260–1268.9. Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM. OPGL is a key regulator ofosteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999. 397:315–323.10. Li J. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogensis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci USA. 2000. 97:1566–1571.11. Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, Wang L, Xia XZ, Elliott R, Chiu L, Black T, Scully S, Capparelli C, Morony S, Shimamoto G, Bass MB, Boyle WJ. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci USA. 1999. 96:3540–3545.12. Bolon B, Carter C, Daris M, Morony S, Capparelli C, Hsieh A, Mao M, Kostenuik P, Dunstan CR, Lacey DL, Sheng JZ. Adenoviral delivery of osteoprotegerin ameliorates bone resorption in a mouse ovariectomy model of osteoporosis. Mol Ther. 2001. 3:197–205.13. Kostenuik PJ, Bolon B, Morony S, Daris M, Geng Z, Carter C, Sheng J. Gene therapy with human recombinant osteoprotegerin reverses established osteopenia inovariectomized mice. Bone. 2004. 34:656–664.14. Nakashima T, Wada T, Penninger JM. RANKL and RANK as novel therapeutic targets for arthritis. Curr Opin Rheumatol. 2003. 15:280–287.15. Hofbauer LC, Neubauer A, Heufelder AE. Receptor activator of nuclear factor-κB ligand and osteoprotegerin; potential implications for the pathogenesis and treatment of malignant bone disease. Cancer. 2001. 92:460–470.16. Zhang J, Dai J, Qi Y, Lin DL, Smith P, Strayhorn C, Mizokami A, Fu Z, Westman J, Keller ET. Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. J Clin Invest. 2001. 107:1235–1244.17. Body JJ, Greipp P, Coleman RE, Facon T, Geurs F, Fermand JP, Harousseau JL, Lipton A, Mariette X, Williams CD, Nakanishi A, Holloway D, Martin SW, Dunstan CR, Bekker PJ. A phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer. 2003. 97:Suppl 3. 887–892.18. Bekker PJ, Holloway D, Nakanishi A, Arrighi HM, Dunstan CR. The effect of a single dose of osteoprotegerin in postmenopauseal women. J Bone Mineral Res. 2001. 16:348–360.19. Byrn RA, Mordenti J, Lucas C, Smith D, Marsters SA, Johnson JS, Cossum P, Chamow SM, Wurm FM, Gregory T, Groopman JE, Capon DJ. Biological properties of a CD4 immunoadhesion. Nature. 1990. 344:667–670.20. Sordillo EM, Pearse RN. RANK-Fc: a therapeutic antagonist for RANK-L in myeloma. Cancer. 2003. 97:Suppl 3. 802–812.21. Oyajobi BO, Anderson DM, Traianedes K, Williams PJ, Yoneda T, Mundy GR. Therapeutic efficacy of a soluble receptor activator of nuclear factor κB-IgG Fc fusion protein in suppressing bone resorption and hypercalcemia in a model of humoral hypercalcemia of malignancy. Cancer Res. 2001. 61:2572–2578.22. Pearse RN, Sordillo EM, Yaccoby S, Wong BR, Liau DF, Colman N, Michaeli J, Epstein J, Choi Y. Multiple myeloma disrupts the TRANCE/osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression. Proc Natl Acad Sci USA. 2001. 98:11581–11586.23. Emery JG, McDonnell P, Burke MB. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998. 273:14363–14367.24. Shipman CM, Croucher PI. Osteoprotegerin is a soluble decoy receptor for tumor necrosis factor-related apoptosis-inducing ligand/Apo2 ligand and can function as a paracrine survival factor for human myeloma cells. Cancer Res. 2003. 63:912–916.25. Holen I, Croucher PI, Hamdy FC, Eaton CL. Osteoprotegerin (OPG) is a survival factor for human prostate cancer cells. Cancer Res. 2002. 62:1619–1623.26. Ko KS, Lee MH, Koh JJ, Kim SW. Combined administration of plasmids encoding IL-4 and IL-10 prevents the development of autoimmune diabetes in nonobese diabetic mice. Mol Ther. 2001. 4:313–316.27. Allay JA, Dennis JE, Haynesworth SE, Majumdar MK, Clapp DW, Shultz LD, Caplan AI, Gerson SL. LacZ and interleukin-3 expression in vivo after retroviral transduction of marrow-derived human osteogenic mesenchymal progenitors. Hum Gene Ther. 1997. 8:1417–1427.28. Ding LM, Saylors R, Munshi NC. The stromal cell as a vehicle for ex vivo gene transfer. Blood. 1997. 89:446–456.29. Chuah MKL, Brems H, Vanslembrouck V, Collen D, Vandendriessche T. Bone marrow stromal cells as targets for gene therapy of hemophilia A. Hum Gene Ther. 1998. 9:353–365.30. Persons DA, Mehaffey MG, Kaleko M, Nienhuis AW, Vanin EF. An improved method for generating retroviral producer clones for vectors lacking a selectable marker gene. Blood Cells Mol Dis. 1998. 24:167–182.31. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997. 276:71–74.32. Prockop DJ, Gregory CA, Spees JL. One strategy for cell and gene therapy: Harnessing the power of adult stem cells to repair tissues. Proc Natl Acad Sci USA. 2003. 100:Suppl 1. 11917–11923.33. Pereira RF, O'Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, Simon D, Livezey K, Prockop DJ. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci USA. 1998. 95:1142–1147.34. Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001. 19:180–192.35. Krebsbach PH, Zhang K, Malik AK, Kurachi K. Bone marrow stromal cells as a genetic platform for systemic delivery of therapeutic proteins in vivo: human factor IX model. J Gene Med. 2003. 5:11–17.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- RANK-Fc Gene Therapy in Mouse Model of Osteoporosis
- A Vitronectin-Derived Peptide Restores Ovariectomy-Induced Bone Loss by Dual Regulation of Bone Remodeling
- Interleukin-10-Producing B Cells Help Suppress Ovariectomy-Mediated Osteoporosis
- The Effect of Intermittent Artificial Gravity (IAG) on Osteoporosis Induced by Ovariectomy in Rats - Histomorphometric Analysis by Using 3D Micro-CT -
- Vitronectin regulates osteoclastogenesis and bone remodeling in a mouse model of osteoporosis