J Lung Cancer.
2008 Dec;7(2):59-64. 10.6058/jlc.2008.7.2.59.
Alterations of the Apoptosis Genes and Their Products in Non-small Cell Lung Cancer Tissues
- Affiliations
-
- 1Department of Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea. suhulee@catholic.ac.kr
- KMID: 2200027
- DOI: http://doi.org/10.6058/jlc.2008.7.2.59
Abstract
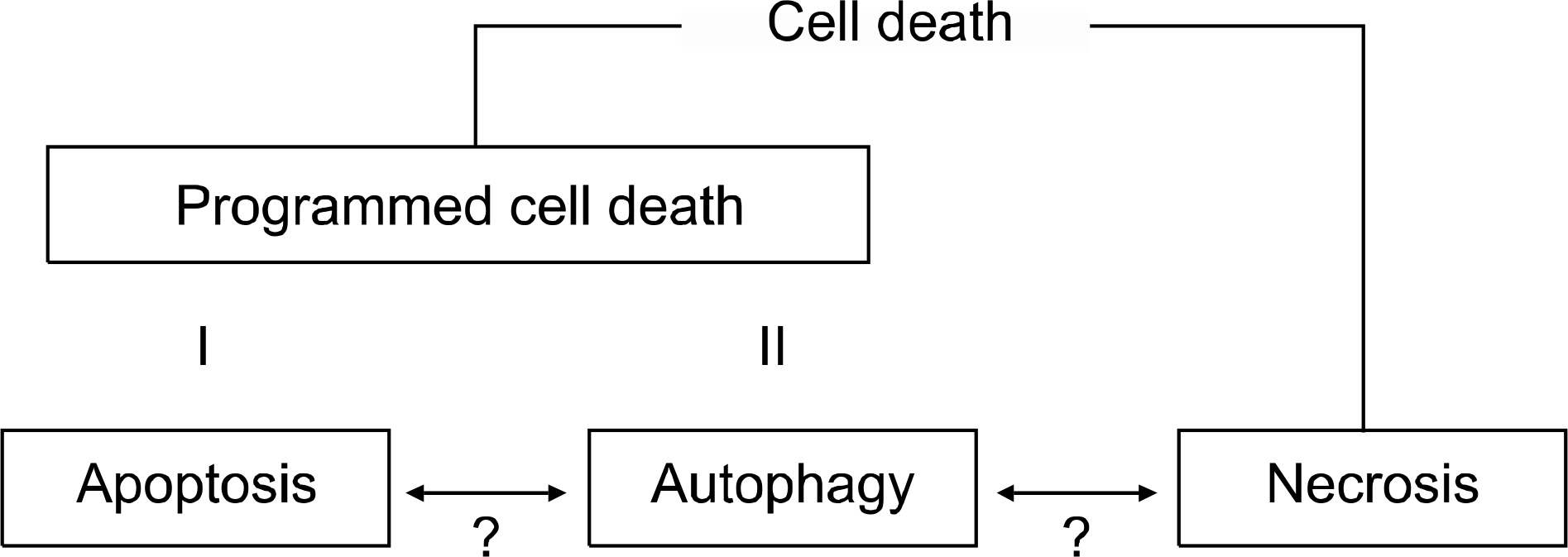
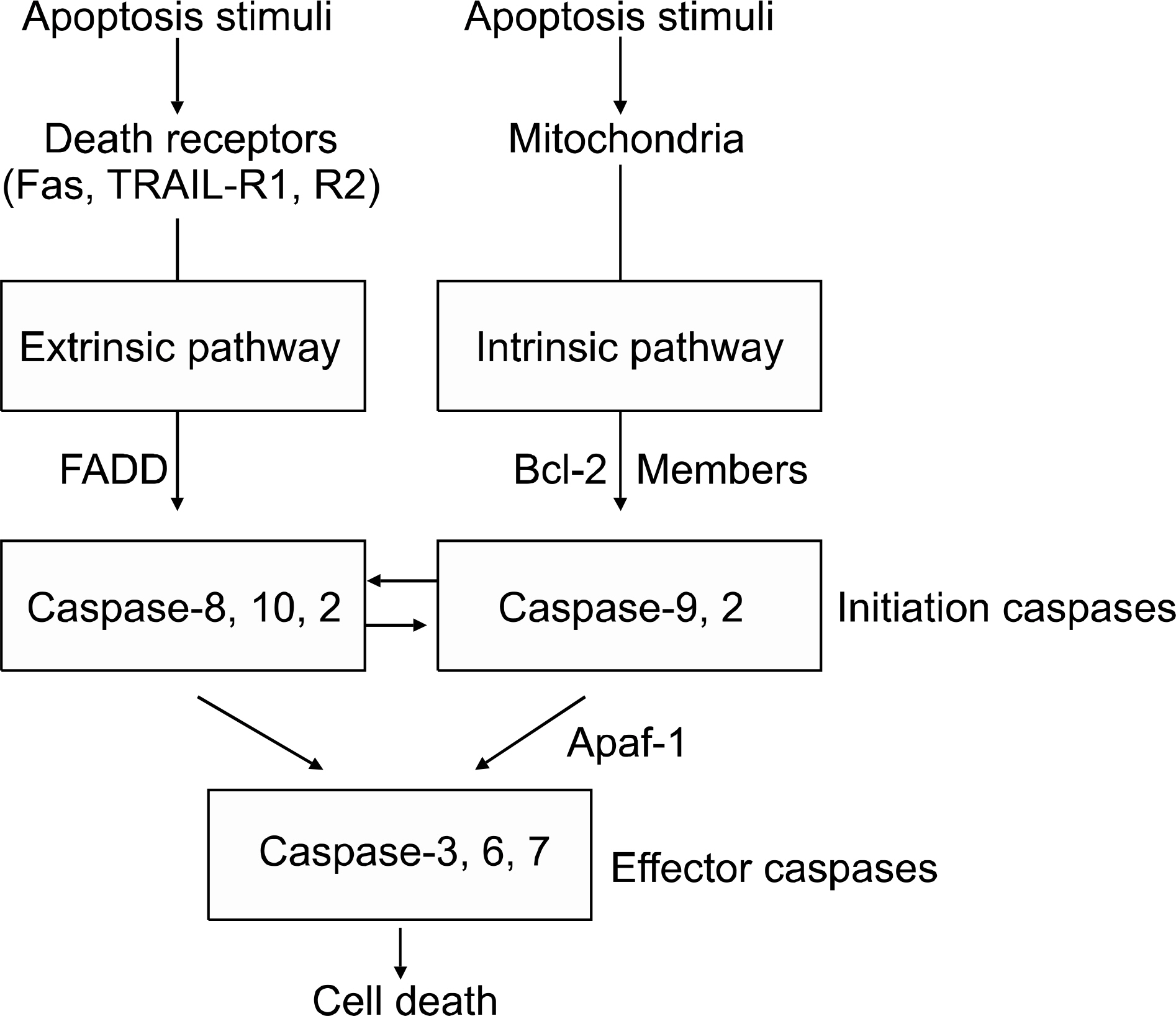
- Apoptosis is a principal type of cell death, and it has a profound effect on the development of cancer. It is also well known that anti-cancer agents induce apoptosis, and defects in the apoptosis pathways reduce the treatment sensitivity. Of the many pathways that induce apoptosis, the mechanisms of the intrinsic and extrinsic apoptosis pathways are well established. Non-small cell lung cancer (NSCLC) is a leading cause of cancer death worldwide, yet the exact molecular mechanisms of its development remain unclear. Apoptosis deregulations may underlie the development and pathogenesis of NSCLC. This review discusses the general mechanisms of apoptosis, the constituents of the apoptosis machinery and the alterations of the apoptosis-related genes in NSCLC
Keyword
Figure
Reference
-
References
1. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000; 100:57–70.
Article2. Reed JC. Mechanisms of apoptosis. Am J Pathol. 2000; 157:1415–1430.
Article3. Fennell DA. Caspase regulation in nonsmall cell lung cancer and its potential for therapeutic exploitation. Clin Cancer Res. 2005; 11:2097–2105.
Article4. Lee SH, Shin MS, Park WS, et al. Alterations of Fas (Apo-1/CD95) gene in nonsmall cell lung cancer. Oncogene. 1999; 18:3754–3760.
Article5. Lee SH, Shin MS, Kim HS, et al. Alterations of the DR5/TRAIL receptor 2 gene in nonsmall cell lung cancers. Cancer Res. 1999; 59:5683–5686.6. Soung YH, Lee JW, Kim SY, et al. Somatic mutations of CASP3 gene in human cancers. Hum Genet. 2004; 115:112–115.
Article7. Nambu Y, Hughes SJ, Rehemtulla A, Hamstra D, Orringer MB, Beer DG. Lack of cell surface Fas/APO-1 expression in pulmonary adenocarcinomas. J Clin Invest. 1998; 101:1102–1110.
Article8. Pezzella F, Turley H, Kuzu I, et al. bcl-2 protein in non-small-cell lung carcinoma. N Engl J Med. 1993; 329:690–694.
Article9. Kroemer G, El-deiry WS, Golstein P, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2005; 12(Suppl 2):1463–1467.
Article10. Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972; 26:239–257.
Article11. McDonnell TJ, Deane N, Platt FM, et al. bcl-2-immunoglo-bulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989; 57:79–88.
Article12. Strasser A, Harris AW, Bath ML, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990; 348:331–333.
Article13. Nagata S. Apoptosis by death factor. Cell. 1997; 88:355–365.
Article14. Leithä user F, Dhein J, Mechtersheimer G, et al. Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab Invest. 1993; 69:415–429.15. Beltinger C, Kurz E, Bö hler T, Schrappe M, Ludwig WD, Debatin KM. CD95 (APO-1/Fas) mutations in childhood T-lineage acute lymphoblastic leukemia. Blood. 1998; 91:3943–3951.
Article16. Landowski TH, Qu N, Buyuksal I, Painter JS, Dalton WS. Mutations in the Fas antigen in patients with multiple myeloma. Blood. 1997; 90:4266–4270.
Article17. Grø nbæ k K, Straten PT, Ralfkiaer E, et al. Somatic Fas mutations in non-Hodgkin's lymphoma: association with extranodal disease and autoimmunity. Blood. 1998; 92:3018–3024.18. Lee SH, Shin MS, Park WS, et al. Alterations of Fas (APO-1/CD95) gene in transitional cell carcinomas of urinary bladder. Cancer Res. 1999; 59:3068–3072.19. Shin MS, Park WS, Kim SY, et al. Alterations of Fas (Apo-1/CD95) gene in cutaneous malignant melanoma. Am J Pathol. 1999; 154:1785–1791.
Article20. Shin MS, Kim HS, Lee SH, et al. Alterations of Fas-pathway genes associated with nodal metastasis in nonsmall cell lung cancer. Oncogene. 2002; 21:4129–4136.21. Shin MS, Kim HS, Lee SH, et al. Mutations of tumor necrosis factor-related apoptosis-inducing ligand receptor 1 (TRAIL-R1) and receptor 2 (TRAIL-R2) genes in metastatic breast cancers. Cancer Res. 2001; 61:4942–4946.22. Kondo S, Shinomura Y, Miyazaki Y, et al. Mutations of the bak gene in human gastric and colorectal cancers. Cancer Res. 2000; 60:4328–4330.23. Arena V, Martini M, Luongo M, Capelli A, Larocca LM. Mutations of the BIK gene in human peripheral B-cell lymphomas. Genes Chromosomes Cancer. 2003; 38:91–96.24. Rampino N, Yamamoto H, Ionov Y, et al. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997; 275:967–969.25. Lee JW, Soung YH, Kim SY, et al. Inactivating mutations of proapoptotic Bad gene in human colon cancers. Carcinogenesis. 2004; 25:1371–1376.
Article26. Lee SH, Soung YH, Lee JW, et al. Mutational analysis of Noxa gene in human cancers. APMIS. 2003; 111:599–604.
Article27. Lee JW, Soung YH, Nam SW, Lee JY, Yoo NJ, Lee SH. Mutational analysis of proapoptotic BAD gene in nonsmall cell lung cancer. J Lung Cancer. 2006; 5:35–38.28. Lee SH, Lee SH. Mutational analysis of proapoptotic BNIP3 gene in nonsmall cell lung cancers. J Lung Cancer. 2007; 6:74–77.
Article29. Yoo NJ, Lee JW, Lee SH, Lee SH. Mutational analysis of PUMA gene in nonsmall cell lung cancers. J Lung Cancer. 2006; 5:92–95.30. Moldvay J, Scheid P, Wild P, et al. Predictive survival markers in patients with surgically resected nonsmall cell lung carcinoma. Clin Cancer Res. 2000; 6:1125–1134.31. Fokkema E, Timens W, de Vries EG, et al. Expression and prognostic implications of apoptosis-related proteins in locally unresectable nonsmall cell lung cancers. Lung Cancer. 2006; 52:241–247.
Article32. Gessner C, Liebers U, Kuhn H, et al. BAX and p16INK4A are independent positive prognostic markers for advanced tumour stage of nonsmall cell lung cancer. Eur Respir J. 2002; 19:134–140.33. Schwartz S Jr, Yamamoto H, Navarro M, Maestro M, Reventos J, Perucho M. Frameshift mutations at mono-nucleotide repeats in caspase-5 and other target genes in endometrial and gastrointestinal cancer of the microsatellite mutator phenotype. Cancer Res. 1999; 59:2995–3002.34. Lee JW, Kim MR, Soung YH, et al. Mutational analysis of the CASP6 gene in colorectal and gastric carcinomas. APMIS. 2006; 114:646–650.
Article35. Soung YH, Lee JW, Kim HS, et al. Inactivating mutations of CASPASE-7 gene in human cancers. Oncogene. 2003; 22:8048–8052.
Article36. Kim HS, Lee JW, Soung YH, et al. Inactivating mutations of caspase-8 gene in colorectal carcinomas. Gastroenterology. 2003; 125:708–715.
Article37. Soung YH, Lee JW, Kim SY, et al. CASPASE-8 gene is inactivated by somatic mutations in gastric carcinomas. Cancer Res. 2005; 65:815–821.38. Soung YH, Lee JW, Kim SY, et al. Mutational analysis of proapoptotic caspase-9 gene in common human carcinomas. APMIS. 2006; 114:292–297.
Article39. Shin MS, Kim HS, Kang CS, et al. Inactivating mutations of CASP10 gene in non-Hodgkin lymphomas. Blood. 2002; 99:4094–4099.
Article40. Yoo NJ, Soung YH, Lee SH, Jeong EG, Lee SH. Mutational analysis of caspase-14 gene in common carcinomas. Pathology. 2007; 39:330–333.
Article41. Yoo NJ, Kim HS, Kim SY, et al. Stomach cancer highly expresses both initiator and effector caspases: an immunohistochemical study. APMIS. 2002; 110:825–832.
Article42. Yoo NJ, Lee JW, Kim YJ, et al. Loss of caspase-2, −6 and −7 expression in gastric cancers. APMIS. 2004; 112:330–335.
Article43. Palmerini F, Devilard E, Jarry A, Birg F, Xerri L. Caspase 7 downregulation as an immunohistochemical marker of colonic carcinoma. Hum Pathol. 2001; 32:461–467.
Article44. Krepela E, Prochá zka J, Fiala P, Zatloukal P, Selinger P. Expression of apoptosome pathway-related transcripts in nonsmall cell lung cancer. J Cancer Res Clin Oncol. 2006; 132:57–68.
Article45. Hopkins-Donaldson S, Ziegler A, Kurtz S, et al. Silencing of death receptor and caspase-8 expression in small cell lung carcinoma cell lines and tumors by DNA methylation. Cell Death Differ. 2003; 10:356–364.
Article46. Putt KS, Chen GW, Pearson JM, et al. Small-molecule activation of procaspase-3 to caspase-3 as a personalized anticancer strategy. Nat Chem Biol. 2006; 2:543–550.
Article47. Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005; 435:677–681.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mutational Analysis of Caspase-7 and 8 Genes in Non-small Cell Lung Cancer
- Mutational Analysis of PUMA Gene in Non-small Cell Lung Cancers
- Mitochondrial Ribosomal Protein S17 Silencing Inhibits Proliferation and Invasiveness of Lung Cancer Cells
- Mutational Analysis of Pro-apoptotic BAD Gene in Nonsmall Cell Lung Cancer
- Targeted Therapy for Non-Small Cell Lung Cancer