J Korean Orthop Assoc.
2008 Jun;43(3):269-275. 10.4055/jkoa.2008.43.3.269.
The Effect of Repeated Intraarticular Bleeding in the Synovium and Articular Cartilage: An Animal Model
- Affiliations
-
- 1Department of Orthopedic Surgery, College of Medicine, Kyung Hee University, Seoul, Korea.
- 2Department of Rheumatology and Hemophilia Research Center, College of Medicine, Kyung Hee University, Seoul, Korea. yhira@khu.ac.kr
- KMID: 2186441
- DOI: http://doi.org/10.4055/jkoa.2008.43.3.269
Abstract
-
PURPOSE: We designed this study to demonstrate the pathophysiology of hemophilic arthropathy (HA) by creating an animal model for determining the effect of repeated intraarticular bleeding in the synovium and articular cartilage.
MATERIALS AND METHODS
20 normal male New Zealand white rabbits were used for this study. We injected 1 ml of autologous blood from the ear vein of the rabbits into the right knee joint three timeds a week for 18 weeks, and we injected 1 ml of normal saline into the left knee joint three times a week for 18 weeks as a control group. We examined the pathologic changes by microscopy and plain X-ray, and we determined the mRNA expression of proinflammatory cytokines in the synovium of the HA by performing real time RT-PCR at the 11th week and 18th week after starting blood-injection. We also examined the GAG and the PGE2 production in cultured chondrocytes that were extracted from the HA knees.
RESULTS
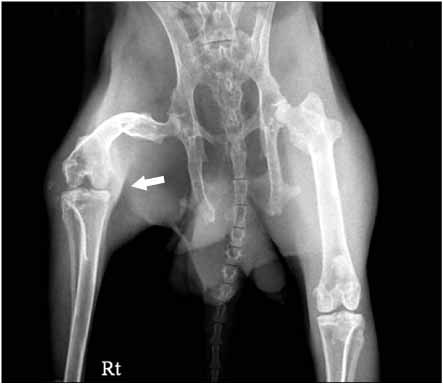
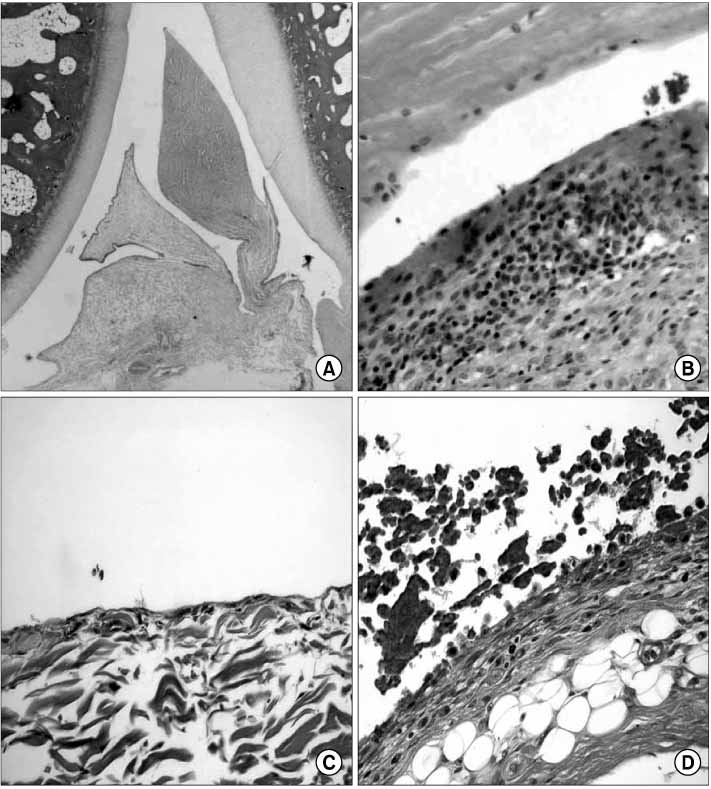
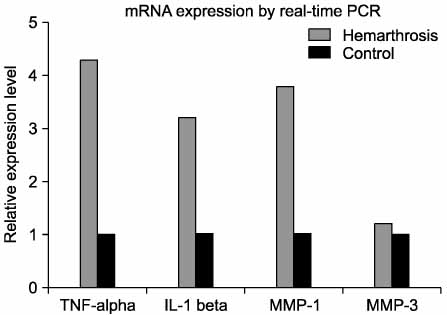
At the 11th week, after blood injection there were no remarkable gross changes in the HA knees and the control knee joints. At the 18th weeks, the experimental knee joints (HA knees) showed grossly swelling and degenerative changes by X-ray. The infiltration of inflammatory cells and the synovial proliferation in the HA knee joints were compared with that in the control knee joints by microscopic examination. The expressions of the mRNA of TNF-alpha, IL-1, MMP-1 and MMP-3 in the HA synovium were increased, as determined by real time RT- PCR, as compared with that in the control knee. In the cultured chondrocytes, the GAG production was decreased and the PGE2 was increased, but the MMP-1 and MMP-3 were not changed, as determined by ELISA.
CONCLUSION
Our results showed that the GAG production of chondrocytes of the HA knees was decreased and there was increased PGE2, so that the cartilage degeneration by intra-articular bleeding was caused by the decreased metabolism of chondrocytes rather than by increased catabolism of the chondrocytes. We suggest that HA was associated with synovitis and cartilage degeneration, but decreased cartilage metabolism was the major mechanism of HA.
MeSH Terms
-
Animals
Cartilage
Chondrocytes
Cytokines
Dinoprostone
Ear
Hemorrhage
Humans
Interleukin-1
Knee
Knee Joint
Male
Microscopy
Models, Animal
Polymerase Chain Reaction
Rabbits
RNA, Messenger
Synovial Membrane
Synovitis
Tumor Necrosis Factor-alpha
Veins
Cytokines
Dinoprostone
Interleukin-1
RNA, Messenger
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Aledort LM, Haschmeyer RH, Pettersson H. A longitudinal study of orthopaedic outcomes for severe factor-VIII-deficient haemophiliacs. The Orthopaedic Outcome Study Group. J Intern Med. 1994. 236:391–399.2. Arnold WD, Hilgartner MW. Hemophilic arthropathy. Current concepts of pathogenesis and management. J Bone Joint Surg Am. 1977. 59:287–305.
Article3. Hooiveld MJ, Roosendaal G, Jacobs KM, et al. Initiation of degenerative joint damage by experimental bleeding combined with loading of the joint: a possible mechanism of hemophilic arthropathy. Arthritis Rheum. 2004. 50:2024–2031.
Article4. Madhok R, Bennett D, Sturrock RD, Forbes CD. Mechanisms of joint damage in an experimental model of hemophilic arthritis. Arthritis Rheum. 1988. 31:1148–1155.
Article5. Madhok R, York J, Sturrock RD. Haemophilic arthritis. Ann Rheum Dis. 1991. 50:588–591.
Article6. Mankin HJ, Dorfman H, Lippeillo L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971. 53:523–537.7. Nishiya K. Stimulation of human synovial cell DNA synthesis by iron. J Rheumatol. 1994. 21:1802–1807.8. Petrini P, Lindvall N, Egberg N, Blombäck M. Prophylaxis with factor concentrates in preventing haemophilic arthopaghy. Am J Peiatrd Hematol Oncol. 1991. 13:280–287.9. Rodriguez-Merchan EC. Methods to treat chronic haemophilic synovitis. Haemophilia. 2001. 7:1–5.
Article10. Rodriguez-Merchán EC. Pathogenesis, early diagnosis, and prophylaxis for chronic hemophilic synovitis. Clin Orthop Relat Res. 1997. 343:6–11.11. Roosendaal G, Mauser-Bunschoten EP, De Kleijn P, et al. Synovium in haemophilic arthropathy. Haemophilia. 1998. 4:502–505.12. Roosendaal G, van Rinsum AC, Vianen ME, van den Berg HM, Lafeber FP, Bijlsma JW. Haemophilic arthropathy resembles degenerative rather than inflammatory joint disease. Histopathology. 1999. 34:144–153.
Article13. Roosendaal G, Vianen ME, Marx JJ, van den Berg HM, Lafeber FP, Bijlsma JW. Blood-induced joint damage: a human in vitro study. Arthritis Rheum. 1999. 42:1025–1032.
Article14. Roosendaal G, Vianen ME, van den Berg HM, Lafeber FP, Bijlsma JW. Cartilage damage as a result of hemarthrosis in a human in vitro model. J Rheumatol. 1997. 24:1350–1354.15. Roosendaal G, Vianen ME, Wenting MJ, et al. Iron deposits and catabolic properties of synovial tissue from patients with haemophilia. J Bone Joint Surg Br. 1998. 80:540–545.
Article16. Soreff J, Blombäck M. Arthropathy in children with severe hemophilia A. Acta Paedriatr Scand. 1980. 69:667–673.
Article17. Stein H, Duthie RB. The pathogenesis of chronic haemophilic arthropathy. J Bone Joint Surg Br. 1981. 63:601–609.
Article18. Sokoloff L. Biochemical and physiological aspects of degenerative joint diseases with special reference to haemophilic arthropathy. Ann NY Acad Sci. 1975. 240:285–290.19. Swanton MC. Hemophilic arthropathy in dogs. Lab Invest. 1959. 8:1269–1277.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Experimental Study of Neochondrogenesis with Reversed Intraarticular Periosteal Flap in Rabbits
- Septic Arthritis after Intra-articular Hyaluronic Acid Injections in Patients with Knee Osteoarthritis: A report of two cases
- Effect of Carboxy Methyl Chitosan on Experimental Osteoarthritis in a Rabbit Knee: Carboxy methyl chitosan vs. Hyaluronan
- Autogeous Bone-Articular Cartilage stored within Abdominal Wall
- A study for the change of articular cartilage and synovium of rabbit knee after osmic acid injection