J Gynecol Oncol.
2011 Mar;22(1):44-48. 10.3802/jgo.2011.22.1.44.
Clinical significance of HIF-2alpha immunostaining area in radioresistant cervical cancer
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. bgkim@skku.edu
- 2Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2173585
- DOI: http://doi.org/10.3802/jgo.2011.22.1.44
Abstract
OBJECTIVE
Hypoxia has been established as a key factor influencing the pathophysiology of malignant growth. Hypoxia-induced changes in gene expression are coordinated primarily by hypoxia inducible factor-1 alpha (HIF-1alpha) and HIF-2alpha. The purpose of this study was to determine whether or not HIF-2alpha expression is associated with survival and response to radiation in patients with cervical cancer.
METHODS
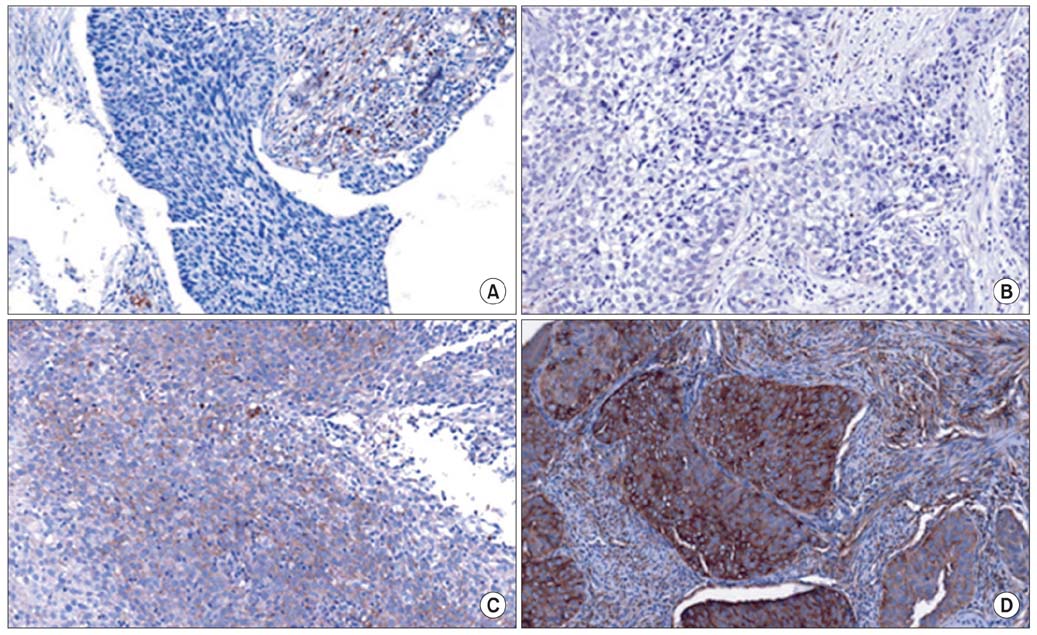
After reviewing the medical records of 119 patients treated in our institution by primary therapy for stage IIB-IVA cervical cancer, we performed a case-control study. Cases (n=12) were selected from patients with local recurrence or radiation failure after primary radiation therapy with or without concurrent chemoradiation. For each case, we selected two controls from patients who had no evidence of local recurrence. Using pre-treatment paraffin-embedded tissues, we evaluated the expression of HIF-2alpha by immunohistochemistry. Staining was scored based on intensity (intensity score [IS], 0-3) and proportion (proportion score [PS], 0-100). The results were analyzed by the Student t-test, Mann-Whitney U test, Fisher's exact test, and Cox proportional hazards regression model.
RESULTS
Cytoplasmic expression of HIF-2alpha, representing the degree of hypoxia, had a relationship with poor response to radiotherapy. The hazard ratio of recurrence was 1.71 for the HIF-2alpha IS (p=0.110) and 1.04 for the HIF-2alpha PS (p<0.001), indicating that the HIF-2alpha staining area correlates weakly with the risk for recurrence.
CONCLUSION
The HIF-2alpha expression area may have an important role in radioresistance in patients with locally advanced cervical cancer. We conclude that a wider area of hypoxia predicts an increased probability of radioresistance.
Keyword
MeSH Terms
Figure
Reference
-
1. Petignat P, Roy M. Diagnosis and management of cervical cancer. BMJ. 2007. 335:765–768.2. Hockel M, Vorndran B, Schlenger K, Baussmann E, Knapstein PG. Tumor oxygenation: a new predictive parameter in locally advanced cancer of the uterine cervix. Gynecol Oncol. 1993. 51:141–149.3. Hockel M, Schlenger K, Mitze M, Schaffer U, Vaupel P. Hypoxia and radiation response in human tumors. Semin Radiat Oncol. 1996. 6:3–9.4. Sutherland RM, Ausserer WA, Murphy BJ, Laderoute KR. Tumor hypoxia and heterogeneity: challenges and opportunities for the future. Semin Radiat Oncol. 1996. 6:59–70.5. Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004. 4:437–447.6. Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955. 9:539–549.7. Ishikawa H, Sakurai H, Hasegawa M, Mitsuhashi N, Takahashi M, Masuda N, et al. Expression of hypoxic-inducible factor 1alpha predicts metastasis-free survival after radiation therapy alone in stage IIIB cervical squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2004. 60:513–521.8. Koukourakis MI, Giatromanolaki A, Sivridis E, Simopoulos C, Turley H, Talks K, et al. Hypoxia-inducible factor (HIF1A and HIF2A), angiogenesis, and chemoradiotherapy outcome of squamous cell head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002. 53:1192–1202.9. Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007. 11:335–347.10. Moeller BJ, Dreher MR, Rabbani ZN, Schroeder T, Cao Y, Li CY, et al. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell. 2005. 8:99–110.11. Bertout JA, Majmundar AJ, Gordan JD, Lam JC, Ditsworth D, Keith B, et al. HIF2alpha inhibition promotes p53 pathway activity, tumor cell death, and radiation responses. Proc Natl Acad Sci U S A. 2009. 106:14391–14396.12. Palayoor ST, Burgos MA, Shoaibi A, Tofilon PJ, Coleman CN. Effect of radiation and ibuprofen on normoxic renal carcinoma cells overexpressing hypoxia-inducible factors by loss of von Hippel-Lindau tumor suppressor gene function. Clin Cancer Res. 2004. 10:4158–4164.13. Franovic A, Holterman CE, Payette J, Lee S. Human cancers converge at the HIF-2alpha oncogenic axis. Proc Natl Acad Sci U S A. 2009. 106:21306–21311.14. Hutchison GJ, Valentine HR, Loncaster JA, Davidson SE, Hunter RD, Roberts SA, et al. Hypoxia-inducible factor 1alpha expression as an intrinsic marker of hypoxia: correlation with tumor oxygen, pimonidazole measurements, and outcome in locally advanced carcinoma of the cervix. Clin Cancer Res. 2004. 10:8405–8412.15. Bachtiary B, Schindl M, Potter R, Dreier B, Knocke TH, Hainfellner JA, et al. Overexpression of hypoxia-inducible factor 1alpha indicates diminished response to radiotherapy and unfavorable prognosis in patients receiving radical radiotherapy for cervical cancer. Clin Cancer Res. 2003. 9:2234–2240.16. Burri P, Djonov V, Aebersold DM, Lindel K, Studer U, Altermatt HJ, et al. Significant correlation of hypoxia-inducible factor-1alpha with treatment outcome in cervical cancer treated with radical radiotherapy. Int J Radiat Oncol Biol Phys. 2003. 56:494–501.17. Kawanaka T, Kubo A, Ikushima H, Sano T, Takegawa Y, Nishitani H. Prognostic significance of HIF-2alpha expression on tumor infiltrating macrophages in patients with uterine cervical cancer undergoing radiotherapy. J Med Invest. 2008. 55:78–86.18. Nakano T, Kato S, Ohno T, Tsujii H, Sato S, Fukuhisa K, et al. Long-term results of high-dose rate intracavitary brachytherapy for squamous cell carcinoma of the uterine cervix. Cancer. 2005. 103:92–101.19. Lee SY, Chung SM. Neovastat (AE-941) inhibits the airway inflammation via VEGF and HIF-2 alpha suppression. Vascul Pharmacol. 2007. 47:313–318.20. Freitas S, Moore DH, Michael H, Kelley MR. Studies of apurinic/apyrimidinic endonuclease/ref-1 expression in epithelial ovarian cancer: correlations with tumor progression and platinum resistance. Clin Cancer Res. 2003. 9:4689–4694.21. Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004. 5:429–441.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulation of glucose metabolism-related genes and VEGF by HIF-1alpha and HIF-1beta, but not HIF-2alpha, in gastric cancer
- Crosstalk between FLS and chondrocytes is regulated by HIF-2alpha-mediated cytokines in arthritis
- Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions
- Nur77 upregulates HIF-alpha by inhibiting pVHL-mediated degradation
- Genipin Inhibits Hypoxia-Induced Accumulation of HIF-1α and VEGF Expressions in Human Cervical Carcinoma Cells