Chonnam Med J.
2012 Aug;48(2):77-85. 10.4068/cmj.2012.48.2.77.
Cysteinyl Cathepsins: Multifunctional Enzymes in Cardiovascular Disease
- Affiliations
-
- 1Department of Cardiology, Yanbian University Hospital, Yanji, Jilin Prov, China. xianwu@med.nagoya-u.ac.jp
- 2Department of Clinical Nutrition, Jiangsu Province Hospital of TCM, Nanjing, China.
- 3Department of Neuogenetics, Nagoya University Graduate School of Medicine, Nagoya, Japan.
- 4Department of Cardiology, Nagoya University Graduate School of Medicine, Nagoya, Japan.
- 5Department of Internal Medicine, Kyung Hee University Hospital, Seoul, Korea.
- KMID: 2172207
- DOI: http://doi.org/10.4068/cmj.2012.48.2.77
Abstract
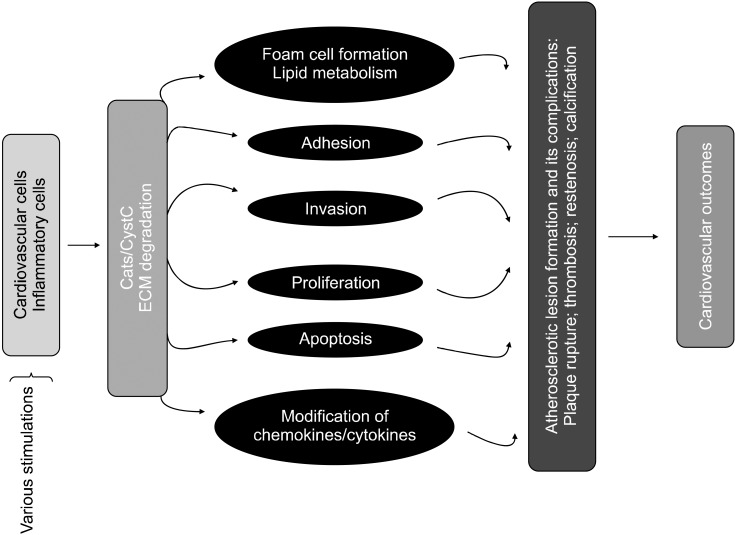
- Until recently, the role of lysosomal cysteine protease cathepsins in intracellular protein degradation was believed to be mainly restricted to scavenging. However, recent studies have revealed nontraditional roles for cysteine protease cathepsins in the extracellular space during the development and progression of cardiovascular disease. Although the precise mechanisms are unknown, data from animal studies suggest that members of the cathepsin family, like other extracellular proteases, contribute to extracellular matrix protein remodeling and interstitial matrix degradation, as well as to cell signaling and cell apoptosis in heart disease. Inflammatory cytokines and hormones regulate the expression and secretion of cathepsins in cultured cardiovascular cells and macrophages. Serum levels of cathepsins L, S, and K and their endogenous inhibitor cystatin C may be useful predictive biomarkers in patients with coronary artery disease and cardiac disease. Furthermore, in vivo pharmacological intervention with a synthetic cathepsin inhibitor and cardiovascular drugs (including statins and angiotensin II type 1 receptor antagonists) has the potential for pharmacologic targeting of cathepsins in cardiovascular disease. This review focuses on cathepsin biology (structure, synthesis, processing, activation, secretion, activity regulation, and function) and the involvement of cysteinyl cathepsins in the pathogenesis of several heart and vessel diseases, especially with respect to their potential application as diagnostic and prognostic markers and drug targets to prevent inappropriate proteolysis in cardiovascular disease.
Keyword
MeSH Terms
-
Animals
Apoptosis
Biomarkers
Biology
Cardiovascular Agents
Cardiovascular Diseases
Cathepsins
Coronary Artery Disease
Cystatin C
Cysteine Proteases
Cytokines
Extracellular Matrix
Extracellular Matrix Proteins
Extracellular Space
Glycosaminoglycans
Heart
Heart Diseases
Humans
Macrophages
Peptide Hydrolases
Proteolysis
Receptor, Angiotensin, Type 1
Cardiovascular Agents
Cathepsins
Cystatin C
Cysteine Proteases
Cytokines
Extracellular Matrix Proteins
Glycosaminoglycans
Peptide Hydrolases
Receptor, Angiotensin, Type 1
Figure
Cited by 1 articles
-
Increased Serum Cathepsin K in Patients with Coronary Artery Disease
Xiang Li, Yuzi Li, Jiyong Jin, Dehao Jin, Lan Cui, Xiangshan Li, Yanna Rei, Haiying Jiang, Guangxian Zhao, Guang Yang, Enbo Zhu, Yongshan Nan, Xianwu Cheng
Yonsei Med J. 2014;55(4):912-919. doi: 10.3349/ymj.2014.55.4.912.
Reference
-
1. Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007; 87:1285–1342. PMID: 17928585.2. Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007; 117:568–575. PMID: 17332884.3. Lutgens SP, Cleutjens KB, Daemen MJ, Heeneman S. Cathepsin cysteine proteases in cardiovascular disease. FASEB J. 2007; 21:3029–3041. PMID: 17522380.4. Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006; 69:562–573. PMID: 16405877.5. Cheng XW, Huang Z, Kuzuya M, Okumura K, Murohara T. Cysteine protease cathepsins in atherosclerosis-based vascular disease and its complications. Hypertension. 2011; 58:978–986. PMID: 21986502.6. van Hinsbergh VW, Engelse MA, Quax PH. Pericellular proteases in angiogenesis and vasculogenesis. Arterioscler Thromb Vasc Biol. 2006; 26:716–728. PMID: 16469948.7. Lemarié CA, Tharaux PL, Lehoux S. Extracellular matrix alterations in hypertensive vascular remodeling. J Mol Cell Cardiol. 2010; 48:433–439. PMID: 19837080.8. Cheng XW, Shi GP, Kuzuya M, Sasaki T, Okumura K, Murohara T. Role for cysteine protease cathepsins in heart disease: focus on biology and mechanisms with clinical implication. Circulation. 2012; 125:1551–1562. PMID: 22451605.9. Carmeliet P, Moons L, Lijnen R, Baes M, Lemaître V, Tipping P, et al. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat Genet. 1997; 17:439–444. PMID: 9398846.10. Cheng XW, Song H, Sasaki T, Hu L, Inoue A, Bando YK, et al. Angiotensin type 1 receptor blocker reduces intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Hypertension. 2011; 57:981–989. PMID: 21464389.11. Kuzuya M, Nakamura K, Sasaki T, Cheng XW, Itohara S, Iguchi A. Effect of MMP-2 deficiency on atherosclerotic lesion formation in apoE-deficient mice. Arterioscler Thromb Vasc Biol. 2006; 26:1120–1125. PMID: 16556856.12. Kuzuya M, Kanda S, Sasaki T, Tamaya-Mori N, Cheng XW, Itoh T, et al. Deficiency of gelatinase a suppresses smooth muscle cell invasion and development of experimental intimal hyperplasia. Circulation. 2003; 108:1375–1381. PMID: 12939223.13. Sun M, Chen M, Liu Y, Fukuoka M, Zhou K, Li G, et al. Cathepsin-L contributes to cardiac repair and remodelling post-infarction. Cardiovasc Res. 2011; 89:374–383. PMID: 21147810.14. Liu J, Sukhova GK, Sun JS, Xu WH, Libby P, Shi GP. Lysosomal cysteine proteases in atherosclerosis. Arterioscler Thromb Vasc Biol. 2004; 24:1359–1366. PMID: 15178558.15. Turk B, Turk V, Turk D. Structural and functional aspects of papain-like cysteine proteinases and their protein inhibitors. Biol Chem. 1997; 378:141–150. PMID: 9165064.16. Rawlings ND, Morton FR, Kok CY, Kong J, Barrett AJ. MEROPS: the peptidase database. Nucleic Acids Res. 2008; 36:D320–D325. PMID: 17991683.17. Coulombe R, Grochulski P, Sivaraman J, Ménard R, Mort JS, Cygler M. Structure of human procathepsin L reveals the molecular basis of inhibition by the prosegment. EMBO J. 1996; 15:5492–5503. PMID: 8896443.18. Sol-Church K, Picerno GN, Stabley DL, Frenck J, Xing S, Bertenshaw GP, et al. Evolution of placentally expressed cathepsins. Biochem Biophys Res Commun. 2002; 293:23–29. PMID: 12054558.19. Reiser J, Adair B, Reinheckel T. Specialized roles for cysteine cathepsins in health and disease. J Clin Invest. 2010; 120:3421–3431. PMID: 20921628.20. Dubin G. Proteinaceous cysteine protease inhibitors. Cell Mol Life Sci. 2005; 62:653–669. PMID: 15770418.21. Baici A, Müntener K, Willimann A, Zwicky R. Regulation of human cathepsin B by alternative mRNA splicing: homeostasis, fatal errors and cell death. Biol Chem. 2006; 387:1017–1021. PMID: 16895470.22. Turk B, Turk D, Turk V. Lysosomal cysteine proteases: more than scavengers. Biochim Biophys Acta. 2000; 1477:98–111. PMID: 10708852.23. Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer. 2006; 6:764–775. PMID: 16990854.24. Cheng XW, Obata K, Kuzuya M, Izawa H, Nakamura K, Asai E, et al. Elastolytic cathepsin induction/activation system exists in myocardium and is upregulated in hypertensive heart failure. Hypertension. 2006; 48:979–987. PMID: 16982960.25. Ge J, Zhao G, Chen R, Li S, Wang S, Zhang X, et al. Enhanced myocardial cathepsin B expression in patients with dilated cardiomyopathy. Eur J Heart Fail. 2006; 8:284–289. PMID: 16480925.26. Sukhova GK, Shi GP, Simon DI, Chapman HA, Libby P. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest. 1998; 102:576–583. PMID: 9691094.27. Sam F, Siwik DA. Digesting the remodeled heart: role of lysosomal cysteine proteases in heart failure. Hypertension. 2006; 48:830–831. PMID: 16982959.28. Jormsjö S, Wuttge DM, Sirsjö A, Whatling C, Hamsten A, Stemme S, et al. Differential expression of cysteine and aspartic proteases during progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2002; 161:939–945. PMID: 12213722.29. Cheng XW, Murohara T, Kuzuya M, Izawa H, Sasaki T, Obata K, et al. Superoxide-dependent cathepsin activation is associated with hypertensive myocardial remodeling and represents a target for angiotensin II type 1 receptor blocker treatment. Am J Pathol. 2008; 173:358–369. PMID: 18583318.30. Aoki T, Kataoka H, Ishibashi R, Nakagami H, Nozaki K, Morishita R, et al. Pitavastatin suppresses formation and progression of cerebral aneurysms through inhibition of the nuclear factor kappaB pathway. Neurosurgery. 2009; 64:357–365. PMID: 19190463.31. Cheng XW, Kuzuya M, Sasaki T, Inoue A, Hu L, Song H, et al. Inhibition of mineralocorticoid receptor is a renoprotective effect of the 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor pitavastatin. J Hypertens. 2011; 29:542–552. PMID: 21119529.32. Liu J, Ma L, Yang J, Ren A, Sun Z, Yan G, et al. Increased serum cathepsin S in patients with atherosclerosis and diabetes. Atherosclerosis. 2006; 186:411–419. PMID: 16140306.33. Liu Y, Li X, Peng D, Tan Z, Liu H, Qing Y, et al. Usefulness of serum cathepsin L as an independent biomarker in patients with coronary heart disease. Am J Cardiol. 2009; 103:476–481. PMID: 19195505.34. Arnlöv J. Cathepsin S as a biomarker: where are we now and what are the future challenges? Biomark Med. 2012; 6:9–11. PMID: 22296192.35. Smith ER, Tomlinson LA, Ford ML, McMahon LP, Rajkumar C, Holt SG. Elastin degradation is associated with progressive aortic stiffening and all-cause mortality in predialysis chronic kidney disease. Hypertension. 2012; 59:973–978. PMID: 22411928.36. Cheng XW, Kuzuya M, Sasaki T, Arakawa K, Kanda S, Sumi D, et al. Increased expression of elastolytic cysteine proteases, cathepsins S and K, in the neointima of balloon-injured rat carotid arteries. Am J Pathol. 2004; 164:243–251. PMID: 14695337.37. Sasaki T, Kuzuya M, Nakamura K, Cheng XW, Hayashi T, Song H, et al. AT1 blockade attenuates atherosclerotic plaque destabilization accompanied by the suppression of cathepsin S activity in apoE-deficient mice. Atherosclerosis. 2010; 210:430–437. PMID: 20079903.38. Liu J, Sukhova GK, Yang JT, Sun J, Ma L, Ren A, et al. Cathepsin L expression and regulation in human abdominal aortic aneurysm, atherosclerosis, and vascular cells. Atherosclerosis. 2006; 184:302–311. PMID: 15982660.39. de Nooijer R, Bot I, von der Thüsen JH, Leeuwenburgh MA, Overkleeft HS, Kraaijeveld AO, et al. Leukocyte cathepsin S is a potent regulator of both cell and matrix turnover in advanced atherosclerosis. Arterioscler Thromb Vasc Biol. 2009; 29:188–194. PMID: 19095996.40. Sukhova GK, Zhang Y, Pan JH, Wada Y, Yamamoto T, Naito M, et al. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003; 111:897–906. PMID: 12639996.41. Rodgers KJ, Watkins DJ, Miller AL, Chan PY, Karanam S, Brissette WH, et al. Destabilizing role of cathepsin S in murine atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2006; 26:851–856. PMID: 16410454.42. Lutgens E, Lutgens SP, Faber BC, Heeneman S, Gijbels MM, de Winther MP, et al. Disruption of the cathepsin K gene reduces atherosclerosis progression and induces plaque fibrosis but accelerates macrophage foam cell formation. Circulation. 2006; 113:98–107. PMID: 16365196.43. Kitamoto S, Sukhova GK, Sun J, Yang M, Libby P, Love V, et al. Cathepsin L deficiency reduces diet-induced atherosclerosis in low-density lipoprotein receptor-knockout mice. Circulation. 2007; 115:2065–2075. PMID: 17404153.44. Lafarge JC, Naour N, Clément K, Guerre-Millo M. Cathepsins and cystatin C in atherosclerosis and obesity. Biochimie. 2010; 92:1580–1586. PMID: 20417681.45. Abdul-Hussien H, Soekhoe RG, Weber E, von der Thüsen JH, Kleemann R, Mulder A, et al. Collagen degradation in the abdominal aneurysm: a conspiracy of matrix metalloproteinase and cysteine collagenases. Am J Pathol. 2007; 170:809–817. PMID: 17322367.46. Cheng XW, Kuzuya M, Nakamura K, Di Q, Liu Z, Sasaki T, et al. Localization of cysteine protease, cathepsin S, to the surface of vascular smooth muscle cells by association with integrin alphanubeta3. Am J Pathol. 2006; 168:685–694. PMID: 16436681.47. Novinec M, Grass RN, Stark WJ, Turk V, Baici A, Lenarcic B. Interaction between human cathepsins K, L, and S and elastins: mechanism of elastinolysis and inhibition by macromolecular inhibitors. J Biol Chem. 2007; 282:7893–7902. PMID: 17227755.48. Shi GP, Sukhova GK, Grubb A, Ducharme A, Rhode LH, Lee RT, et al. Cystatin C deficiency in human atherosclerosis and aortic aneurysms. J Clin Invest. 1999; 104:1191–1197. PMID: 10545518.49. Abisi S, Burnand KG, Humphries J, Waltham M, Taylor P, Smith A. Effect of statins on proteolytic activity in the wall of abdominal aortic aneurysms. Br J Surg. 2008; 95:333–337. PMID: 17968978.50. Sukhova GK, Wang B, Libby P, Pan JH, Zhang Y, Grubb A, et al. Cystatin C deficiency increases elastic lamina degradation and aortic dilatation in apolipoprotein E-null mice. Circ Res. 2005; 96:368–375. PMID: 15653570.51. Sun J, Sukhova GK, Zhang J, Chen H, Sjöberg S, Libby P, et al. Cathepsin L activity is essential to elastase perfusion-induced abdominal aortic aneurysms in mice. Arterioscler Thromb Vasc Biol. 2011; 31:2500–2508. PMID: 21868704.52. Sun J, Sukhova GK, Zhang J, Chen H, Sjöberg S, Libby P, et al. Cathepsin K deficiency reduces elastase perfusion-induced abdominal aortic aneurysms in mice. Arterioscler Thromb Vasc Biol. 2012; 32:15–23. PMID: 21817099.53. Bai L, Beckers L, Wijnands E, Lutgens SP, Herías MV, Saftig P, et al. Cathepsin K gene disruption does not affect murine aneurysm formation. Atherosclerosis. 2010; 209:96–103. PMID: 19775691.54. Shi GP, Sukhova GK, Kuzuya M, Ye Q, Du J, Zhang Y, et al. Deficiency of the cysteine protease cathepsin S impairs microvessel growth. Circ Res. 2003; 92:493–500. PMID: 12600886.55. Wang B, Sun J, Kitamoto S, Yang M, Grubb A, Chapman HA, et al. Cathepsin S controls angiogenesis and tumor growth via matrix-derived angiogenic factors. J Biol Chem. 2006; 281:6020–6029. PMID: 16365041.56. Cheng XW, Kuzuya M, Nakamura K, Maeda K, Tsuzuki M, Kim W, et al. Mechanisms underlying the impairment of ischemia-induced neovascularization in matrix metalloproteinase 2-deficient mice. Circ Res. 2007; 100:904–913. PMID: 17322177.57. Urbich C, Heeschen C, Aicher A, Sasaki K, Bruhl T, Farhadi MR, et al. Cathepsin L is required for endothelial progenitor cell-induced neovascularization. Nat Med. 2005; 11:206–213. PMID: 15665831.58. Shimada N, Ohno-Matsui K, Iseki S, Koike M, Uchiyama Y, Wang J, et al. Cathepsin L in bone marrow-derived cells is required for retinal and choroidal neovascularization. Am J Pathol. 2010; 176:2571–2580. PMID: 20304958.59. Urbich C, Dernbach E, Rössig L, Zeiher AM, Dimmeler S. High glucose reduces cathepsin L activity and impairs invasion of circulating progenitor cells. J Mol Cell Cardiol. 2008; 45:429–436. PMID: 18619973.60. Horlitz M, Sigwart U, Niebauer J. Fighting restenosis after coronary angioplasty: contemporary and future treatment options. Int J Cardiol. 2002; 83:199–205. PMID: 12036521.61. Sopko G. Preventing cardiac events and restenosis after percutaneous coronary intervention. JAMA. 2002; 287:3259–3261. PMID: 12076224.62. Burns-Kurtis CL, Olzinski AR, Needle S, Fox JH, Capper EA, Kelly FM, et al. Cathepsin S expression is up-regulated following balloon angioplasty in the hypercholesterolemic rabbit. Cardiovasc Res. 2004; 62:610–620. PMID: 15158154.63. Sasaki T, Kuzuya M, Nakamura K, Cheng XW, Shibata T, Sato K, et al. A simple method of plaque rupture induction in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2006; 26:1304–1309. PMID: 16574894.64. Rekhter MD. How to evaluate plaque vulnerability in animal models of atherosclerosis? Cardiovasc Res. 2002; 54:36–41. PMID: 12062359.65. Jackson CL, Bennett MR, Biessen EA, Johnson JL, Krams R. Assessment of unstable atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2007; 27:714–720. PMID: 17332492.66. Nakamura K, Sasaki T, Cheng XW, Iguchi A, Sato K, Kuzuya M. Statin prevents plaque disruption in apoE-knockout mouse model through pleiotropic effect on acute inflammation. Atherosclerosis. 2009; 206:355–361. PMID: 19296953.67. Bouvet C, Moreau S, Blanchette J, de Blois D, Moreau P. Sequential activation of matrix metalloproteinase 9 and transforming growth factor beta in arterial elastocalcinosis. Arterioscler Thromb Vasc Biol. 2008; 28:856–862. PMID: 18292396.68. Qin X, Corriere MA, Matrisian LM, Guzman RJ. Matrix metalloproteinase inhibition attenuates aortic calcification. Arterioscler Thromb Vasc Biol. 2006; 26:1510–1516. PMID: 16690876.69. Aikawa E, Aikawa M, Libby P, Figueiredo JL, Rusanescu G, Iwamoto Y, et al. Arterial and aortic valve calcification abolished by elastolytic cathepsin S deficiency in chronic renal disease. Circulation. 2009; 119:1785–1794. PMID: 19307473.70. Cheng XW, Zhang J, Song H, Yang G, Qin XZ, Guan LK, et al. Association between lysosomal cysteine protease cathepsin's activation and left ventricular function and remodeling in hypertensive heart failure rats. Zhonghua Xin Xue Guan Bing Za Zhi. 2008; 36:51–56. PMID: 19099930.71. Beers C, Honey K, Fink S, Forbush K, Rudensky A. Differential regulation of cathepsin S and cathepsin L in interferon gamma-treated macrophages. J Exp Med. 2003; 197:169–179. PMID: 12538657.72. Gerszten RE, Tager AM. The monocyte in atherosclerosis--should I stay or should I go now? N Engl J Med. 2012; 366:1734–1736. PMID: 22551134.73. Pauly RR, Passaniti A, Bilato C, Monticone R, Cheng L, Papadopoulos N, et al. Migration of cultured vascular smooth muscle cells through a basement membrane barrier requires type IV collagenase activity and is inhibited by cellular differentiation. Circ Res. 1994; 75:41–54. PMID: 8013081.74. Sun J, Zhang J, Lindholt JS, Sukhova GK, Liu J, He A, et al. Critical role of mast cell chymase in mouse abdominal aortic aneurysm formation. Circulation. 2009; 120:973–982. PMID: 19720934.75. Kuzuya M, Cheng XW, Sasaki T, Tamaya-Mori N, Iguchi A. Pitavastatin, a 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor, blocks vascular smooth muscle cell populated-collagen lattice contraction. J Cardiovasc Pharmacol. 2004; 43:808–814. PMID: 15167274.76. Bendeck MP, Zempo N, Clowes AW, Galardy RE, Reidy MA. Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ Res. 1994; 75:539–545. PMID: 8062427.77. Yang M, Sun J, Zhang T, Liu J, Zhang J, Shi MA, et al. Deficiency and inhibition of cathepsin K reduce body weight gain and increase glucose metabolism in mice. Arterioscler Thromb Vasc Biol. 2008; 28:2202–2208. PMID: 18818416.78. Samokhin AO, Lythgo PA, Gauthier JY, Percival MD, Brömme D. Pharmacological inhibition of cathepsin S decreases atherosclerotic lesions in Apoe-/- mice. J Cardiovasc Pharmacol. 2010; 56:98–105. PMID: 20410833.79. Suganuma E, Babaev VR, Motojima M, Zuo Y, Ayabe N, Fogo AB, et al. Angiotensin inhibition decreases progression of advanced atherosclerosis and stabilizes established atherosclerotic plaques. J Am Soc Nephrol. 2007; 18:2311–2319. PMID: 17634441.80. Cheng XW, Okumura K, Kuzuya M, Jin Z, Nagata K, Obata K, et al. Mechanism of diastolic stiffening of the failing myocardium and its prevention by angiotensin receptor and calcium channel blockers. J Cardiovasc Pharmacol. 2009; 54:47–56. PMID: 19528815.81. Stypmann J, Gläser K, Roth W, Tobin DJ, Petermann I, Matthias R, et al. Dilated cardiomyopathy in mice deficient for the lysosomal cysteine peptidase cathepsin L. Proc Natl Acad Sci USA. 2002; 99:6234–6239. PMID: 11972068.82. Petermann I, Mayer C, Stypmann J, Biniossek ML, Tobin DJ, Engelen MA, et al. Lysosomal, cytoskeletal, and metabolic alterations in cardiomyopathy of cathepsin L knockout mice. FASEB J. 2006; 20:1266–1268. PMID: 16636100.83. Taleb S, Lacasa D, Bastard JP, Poitou C, Cancello R, Pelloux V, et al. Cathepsin S, a novel biomarker of adiposity: relevance to atherogenesis. FASEB J. 2005; 19:1540–1542. PMID: 15985526.84. Zhang J, Wang P, Huang YB, Li J, Zhu J, Luo X, et al. Plasma cathepsin L and its related pro/antiangiogenic factors play useful roles in predicting rich coronary collaterals in patients with coronary heart disease. J Int Med Res. 2010; 38:1389–1403. PMID: 20926012.85. Takeuchi T, Isobe S, Sato K, Kato MI, Kasai NN, Ohyama H, et al. Cystatin C: a possible sensitive marker for detecting potential kidney injury after computed tomography coronary angiography. J Comput Assist Tomogr. 2011; 35:240–245. PMID: 21412097.86. Alehagen U, Dahlström U, Lindahl TL. Cystatin C and NT-proBNP, a powerful combination of biomarkers for predicting cardiovascular mortality in elderly patients with heart failure: results from a 10-year study in primary care. Eur J Heart Fail. 2009; 11:354–360. PMID: 19228797.87. Patel PC, Ayers CR, Murphy SA, Peshock R, Khera A, de Lemos JA, et al. Association of cystatin C with left ventricular structure and function: the Dallas Heart Study. Circ Heart Fail. 2009; 2:98–104. PMID: 19808324.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of common bile duct ligation on liver cathepsins B, D, H and acid phosphatase activities in ethanol intoxicated rats
- Potential role(s) of cysteine cathepsins in cancer progression and metastasis
- Significance of the Expression of Cathepsins B, H, & L in Colonic Epithelial Neoplasms
- The Effects of Combination Therapy of Cathepsin K Inhibitor and PTH on Change of Bone Mineral Density in Animal Model of Osteoporosis
- Letter: The Effects of Combination Therapy of Cathepsin K Inhibitor and PTH on Change in Bone Mineral Density in an Animal Model of Osteoporosis