Endocrinol Metab.
2010 Sep;25(3):192-198. 10.3803/EnM.2010.25.3.192.
The Effect of Atorvastatin and Simvastatin on NIS Expression of the TPC-1 Cell under the Therapeutic Blood Concentrations
- Affiliations
-
- 1Department of Internal Medicine, Pusan Paik Hospital, College of Medicine, Inje University, Busan, Korea. pjhdoc@chol.com
- 2Molecular Therapy Lab., Paik Memorial Institute for Clinical Research, Inje University, Busan, Korea.
- 3Department of Internal Medicine, Marynoll Medical Center, Busan, Korea.
- KMID: 2169056
- DOI: http://doi.org/10.3803/EnM.2010.25.3.192
Abstract
- BACKGROUND
Although so many experimental trials have been done to improve the redifferentiation and responsiveness of radioiodide therapy, they have not yet yielded any satisfactory results. As statins inhibit both farnesylation and geranylgeranylation, they have been reported to have an antineoplastic and redifferentiation effect in experimental and clinical studies. In this study, we investigated the relationship between statins and the alteration of the NIS expression and, TPC-1 cell apotosis to evaluate the possibility of using statins as adjuvant therapeutic agents for papillary thyroid cancer.
METHODS
We used the TPC-1 cell lines for our experiments. Cell viabilities were measured by CCK-8. The degrees of apoptosis and, the expressions of NIS mRNA and NIS protein were measured by flow cytometry, semi quantitative RT-PCR and Western blot assay.
RESULTS
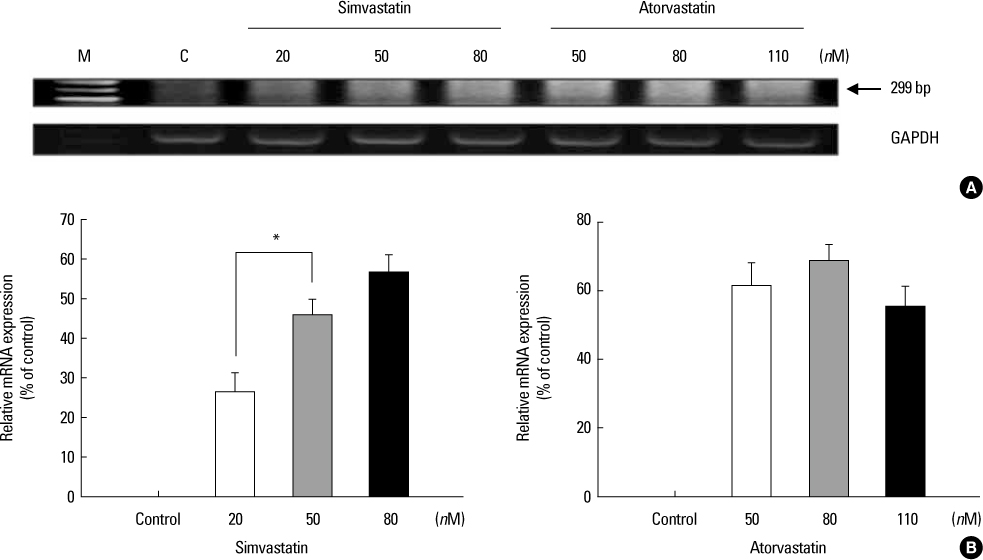
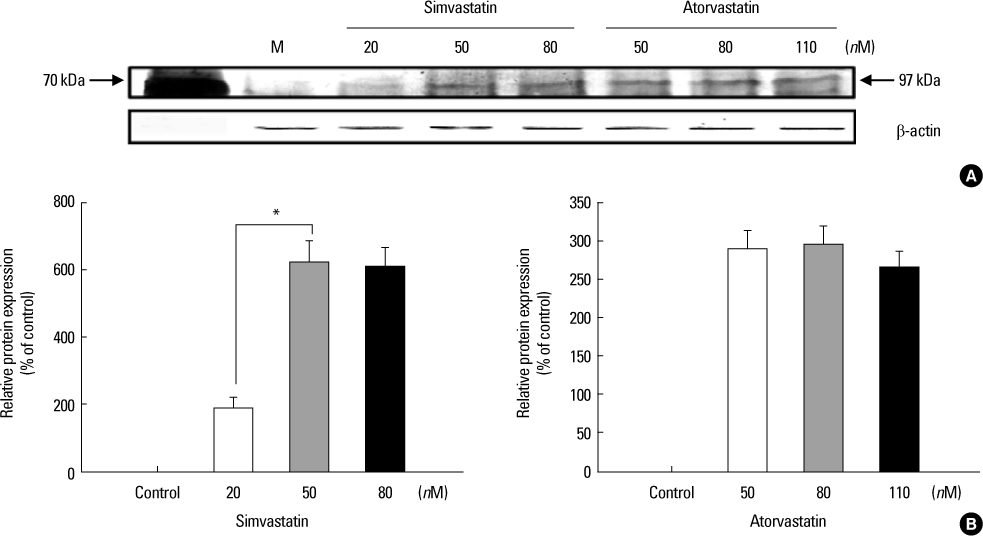
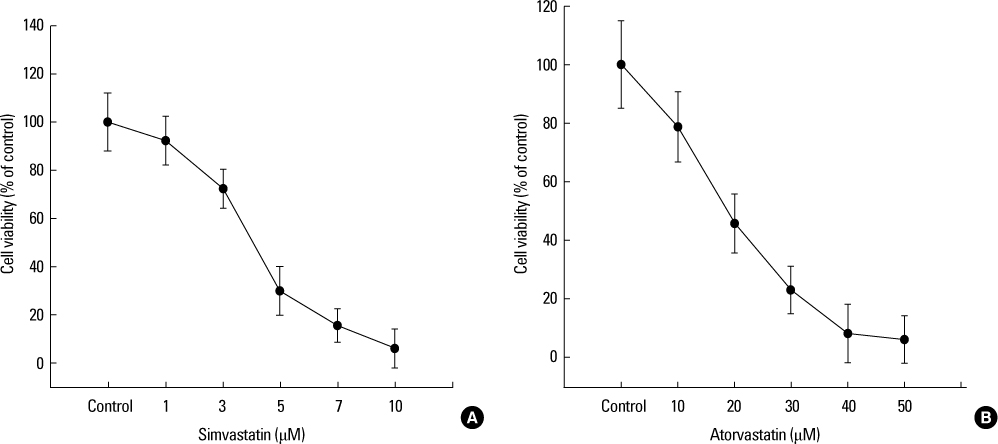
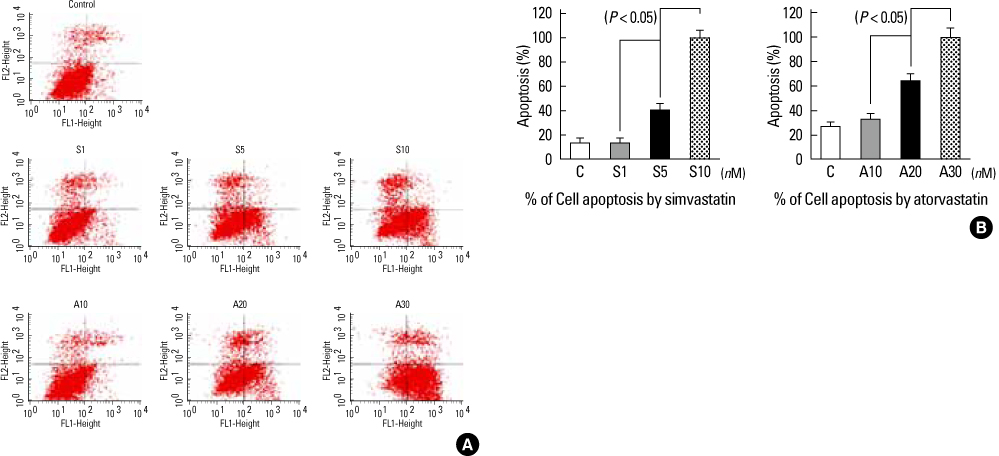
Increased levels of NIS mRNA and NIS protein were observed under therapeutic blood concentrations (concentrations of simvastatin: 20, 50, 80 nM, concentrations of atorvastatin: 50, 80,110 nM), but the dose-response relationship was only manifested within simvastatin. The TPC-1 cells showed a concentration dependent decrease of viability and an increase of apoptosis not under therapeutic blood concentrations, but under excessively high concentrations (after treatment with 10-50 microM of atorvastatin and with 1-10 microM of simvastatin).
CONCLUSION
The results of this study show that effective therapeutic blood concentrations of simvastatin and atorvastatin can give a favorable effect on the NIS expression under effective therapeutic blood concentrations. Therefore, we demonstrated the possibility that simvastatin and atorvastatin might have an important role as adjuvant therapeutic agents to improve the responsiveness of radioiodide therapy for papillary thyroid cancer. Further studies are needed to clarify this issue.
Keyword
MeSH Terms
-
Apoptosis
Blotting, Western
Cell Line
Cell Survival
Flow Cytometry
Heptanoic Acids
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Prenylation
Pyrroles
RNA, Messenger
Simvastatin
Sincalide
Symporters
Thyroid Neoplasms
Atorvastatin Calcium
Heptanoic Acids
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Pyrroles
RNA, Messenger
Simvastatin
Sincalide
Symporters
Figure
Reference
-
1. Min JJ, Chung JK, Lee YJ, Jeong JM, Lee DS, Jang JJ, Lee MC, Cho BY. Relationship between expression of the sodium/iodide symporter and 131I uptake in recurrent lesions of differentiated thyroid carcinoma. Eur J Nucl Med. 2001. 28:639–645.2. Romei C, Ciampi R, Faviana P, Agate L, Molinaro E, Bottici V, Basolo F, Miccoli P, Pacini F, Pinchera A, Elisei R. BRAFV600E mutation, but not RET/PTC rearrangements, is correlated with a lower expression of both thyroperoxidase and sodium iodide symporter genes in papillary thyroid cancer. Endocr Relat Cancer. 2008. 15:511–520.3. Boudreau DM, Yu O, Miglioretti DL, Buist DS, Heckbert SR, Daling JR. Statin use and breast cancer risk in a large population-based setting. Cancer Epidemiol Biomarkers Prev. 2007. 16:416–421.4. Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res. 2003. 9:10–19.5. Shitara Y, Sugiyama Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: drug-drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol Ther. 2006. 112:71–105.6. Choi HJ, Kim TY, Kim EY, Kim WG, Kim WB, Shong YK. Effects of simvastatin on the growth and invasion of anaplastic thyroid cancer cells lines. J Korean Endocr Soc. 2008. 23:238–244.7. Wang CY, Zhong WB, Chang TC, Lai SM, Tsai YF. Lovastatin, a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, induces apoptosis and differentiation in human anaplastic thyroid carcinoma cells. J Clin Endocrinol Metab. 2003. 88:3021–3026.8. Laezza C, Fiorentino L, Pisanti S, Gazzerro P, Caraglia M, Portella G, Vitale M, Bifulco M. Lovastatin induces apoptosis of k-ras-transformed thyroid cells via inhibition of ras farnesylation and by modulating redox state. J Mol Med. 2008. 86:1341–1351.9. Jhiang SM, Cho JY, Furminger TL, Sagartz JE, Tong Q, Capen CC, Mazzaferri EL. Thyroid carcinomas in RET/PTC transgenic mice. Recent Results Cancer Res. 1998. 154:265–270.10. Venkateswaran A, Marsee DK, Green SH, Jhiang SM. Forskolin, 8-Br-3,5-cyclic adenosine 5-monophosphate, and catalytic protein kinase A expression in the nucleus increase radioiodide uptake and sodium/iodide symporter protein levels in RET/PTC-1expressing cells. J Clin Endocrinol Metab. 2004. 89:6168–6172.11. Knauf JA, Kuroda H, Basu S, Fagin JA. RET/PTC-induced dedifferentiation of thyroid cells is mediated through Y1062 signaling through SHC-RAS-MAP kinase. Oncogene. 2003. 22:4406–4412.12. Vadysirisack DD, Venkateswaran A, Zhang Z, Jhiang SM. MEK signaling modulates sodium iodide symporter at multiple levels and in a paradoxical manner. Endocr Relat Cancer. 2007. 14:421–432.13. Nikiforov YE. Thyroid carcinoma: molecular pathways and therapeutic targets. Mod Pathol. 2008. 21:S37–S43.14. Piotrowski PC, Kwintkiewicz J, Rzepczynska IJ, Seval Y, Cakmak H, Arici A, Duleba AJ. Statins inhibit growth of human endometrial stromal cells independently of cholesterol availability. Biol Reprod. 2006. 75:107–111.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Simvastatin on the Expression of VEGF in Human Retinal Pigment Epithelial Cells
- The Antiproliferative and Redifferentiative Effects of Na-4-Phenylbutyrate in Human Thyroid Cancer Cell Lines
- Effects of simvastatin on the proliferation and apoptosis of human endometrial stromal cells from women with endometriosis
- The Effect of Simvastatin on the Expression of Catalase in Human Retinal Pigment Epithelial Cells
- Neuroprotection for Acute Spinal Cord Injury: Comparison of Simvastatin and Atorvastatin