Toll-like Receptors and Innate Immunity
- Affiliations
-
- 1Department of Microbiology, Chungnam National University School of Medicine, Daejeon, Korea. hayoungj@cnu.ac.kr
- 2Infection Signaling Network Research Center, Chungnam National University School of Medicine, Daejeon, Korea.
- KMID: 2168650
- DOI: http://doi.org/10.4167/jbv.2011.41.4.225
Abstract
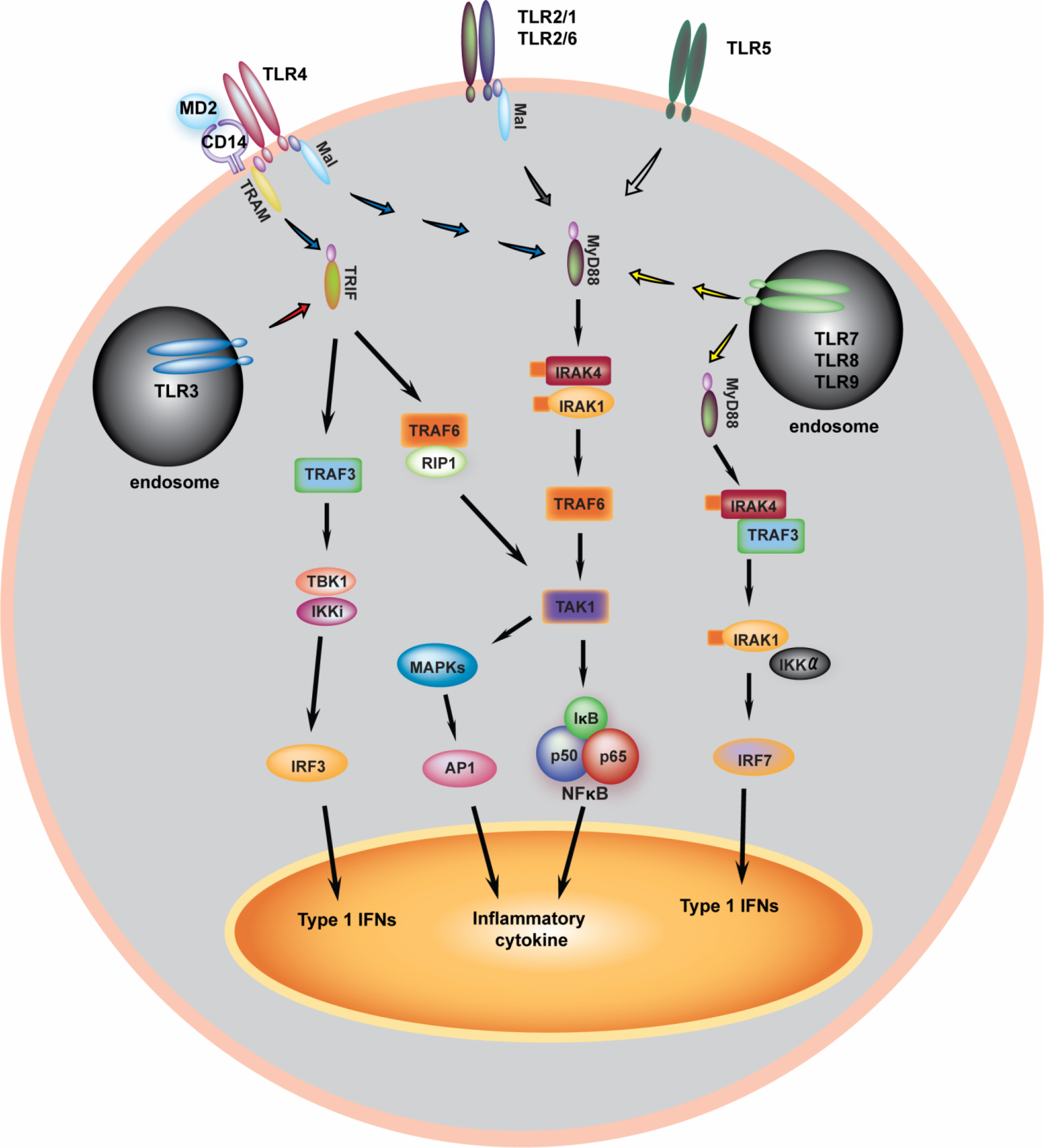
- Toll-like receptors (TLRs) are the best-characterized membrane-bound receptors in innate immune cells, including macrophages and dendritic cells. Upon recognition of specific ligands originating from pathogen- and modified self-derived molecules, TLRs trigger intracellular signaling cascades that involve various adaptor proteins and enzymes, resulting in the generation of proinflammatory and antimicrobial responses through the activation of transcription factors such as nuclear factor-kappaB. TLR-dependent signaling pathways are tightly regulated during innate immune responses by a variety of negative regulators. This review focuses on the newly described regulation of TLR-dependent signaling pathways, and emphasizes the roles of TLRs in innate immunity. Efforts to modulate these regulatory pathways and signaling molecules may result in the development of new therapeutic strategies through TLR-based therapy.
MeSH Terms
Figure
Cited by 12 articles
-
Helicobacter pylori: Bacterial Strategy for Incipient Stage and Persistent Colonization in Human Gastric Niches
Kwang-Ho Rhee, Jin-Sik Park, Myung-Je Cho
Yonsei Med J. 2014;55(6):1453-1466. doi: 10.3349/ymj.2014.55.6.1453.Mitochondrial Control of Innate Immunity and Inflammation
Hyo Sun Jin, Hyun-Woo Suh, Seong-Jun Kim, Eun-Kyeong Jo
Immune Netw. 2017;17(2):77-88. doi: 10.4110/in.2017.17.2.77.Intracellular Signaling Pathways that Regulate Macrophage Chemokine Expression in Response to Mycobacterium abscessus
Tae Sung Kim, Hye-Mi Lee, Heekyung Yoo, Young Kil Park, Eun-Kyeong Jo
J Bacteriol Virol. 2012;42(2):121-132. doi: 10.4167/jbv.2012.42.2.121.Mitogen-activated Protein Kinases in Inflammation
Zahid Manzoor, Young-Sang Koh
J Bacteriol Virol. 2012;42(3):189-195. doi: 10.4167/jbv.2012.42.3.189.Toll-Like Receptor Ligands as Cancer Immunotherapeutics
Shee Eun Lee, Joon Haeng Rhee
J Bacteriol Virol. 2012;42(3):255-262. doi: 10.4167/jbv.2012.42.3.255.Bacterial 23S Ribosomal RNA, a Ligand for Toll-like Receptor 13
Zahid Manzoor, Young-Sang Koh
J Bacteriol Virol. 2012;42(4):357-358. doi: 10.4167/jbv.2012.42.4.357.Anti-inflammatory Activity of Carpinus tschonoskii Leaves Extract in R848-stimulated Bone Marrow-derived Macrophages and Dendritic Cells
Sung-Ha Kang, Jung-Eun Koo, Hye-Jin Hong, Vivek Bhakta Mathema, Young-Sang Koh
J Bacteriol Virol. 2012;42(1):77-82. doi: 10.4167/jbv.2012.42.1.77.Roles of Enteric Microbial Composition and Metabolism in Health and Diseases
Jung Mogg Kim
Korean J Gastroenterol. 2013;62(4):191-205. doi: 10.4166/kjg.2013.62.4.191.The Synergistic Effects of Antimicrobial Peptides on the Growth Inhibition of
Salmonella Typhimurium through Imd Pathway inDrosophila Intestine
Yun-Ji Lim, Yea-Hyeon Jo, Hwa-Jung Kim, Jeong-Kyu Park
J Bacteriol Virol. 2013;43(2):120-130. doi: 10.4167/jbv.2013.43.2.120.Peptidylarginine Deiminase and Citrullination: Potential Therapeutic Targets for Inflammatory Diseases
Byungki Jang, Sung Jae Shin
J Bacteriol Virol. 2013;43(3):159-167. doi: 10.4167/jbv.2013.43.3.159.Sensing DNA Viruses and Bacteria by Intracellular DNA Sensors
Na-Rae Lee, Han-Bo Shin, Hye-In Kim, Myung-Soo Choi, Kyung-Soo Inn
J Bacteriol Virol. 2013;43(2):77-84. doi: 10.4167/jbv.2013.43.2.77.Acrosorium polyneurum Extract Inhibits the LPS-Induced Inflammatory Response by Impairing the MAPK and NF-κB Pathways
Zahid Manzoor, Irshad Ali, Doobyeong Chae, Young-Sang Koh
J Bacteriol Virol. 2016;46(4):288-294. doi: 10.4167/jbv.2016.46.4.288.
Reference
-
1). Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007; 449:819–26.
Article2). Ishii KJ, Koyama S, Nakagawa A, Coban C, Akira S. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe. 2008; 3:352–63.
Article3). Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005; 17:1–14.4). Sun SC, Ley SC. New insights into NF-kappaB regulation and function. Trends Immunol. 2008; 29:469–78.5). Li X, Jiang S, Tapping RI. Toll-like receptor signaling in cell proliferation and survival. Cytokine. 2010; 49:1–9.
Article6). O'Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci STKE. 2000; 2000:re1.7). Shibolet O, Podolsky DK. TLRs in the Gut. I V. Negative regulation of Toll-like receptors and intestinal homeostasis: addition by subtraction. Am J Physiol Gastrointest Liver Physiol. 2007; 292:G1469–73.8). Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996; 86:973–83.9). Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010; 11:373–84.
Article10). Manavalan B, Basith S, Choi S. Similar Structures but Different Roles - An Updated Perspective on TLR Structures. Front Physiol. 2011; 2:41.
Article11). Wang X, Quinn PJ. Lipopolysaccharide: Biosynthetic pathway and structure modification. Prog Lipid Res. 2010; 49:97–107.
Article12). Cohen J. The immunopathogenesis of sepsis. Nature. 2002; 420:885–91.
Article13). Tapping RI, Tobias PS. Mycobacterial lipoarabinomannan mediates physical interactions between TLR1 and TLR2 to induce signaling. J Endotoxin Res. 2003; 9:264–8.
Article14). Goodridge HS, Wolf AJ, Underhill DM. Beta-glucan recognition by the innate immune system. Immunol Rev. 2009; 230:38–50.15). Vijay-Kumar M, Gewirtz AT. Flagellin: key target of mucosal innate immunity. Mucosal Immunol. 2009; 2:197–205.
Article16). Plattner F, Yarovinsky F, Romero S, Didry D, Carlier MF, Sher A, et al. Toxoplasma profilin is essential for host cell invasion and TLR11-dependent induction of an interleukin-12 response. Cell Host Microbe. 2008; 3:77–87.17). Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001; 413:732–8.18). Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002; 3:196–200.
Article19). Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004; 303:1529–31.
Article20). Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004; 303:1526–9.
Article21). Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000; 408:740–5.
Article22). Carty M, Bowie AG. Evaluating the role of Toll-like receptors in diseases of the central nervous system. Biochem Pharmacol. 2011; 81:825–37.
Article23). Jin MS, Lee JO. Structures of the toll-like receptor family and its ligand complexes. Immunity. 2008; 29:182–91.
Article24). Latz E, Verma A, Visintin A, Gong M, Sirois CM, Klein DC, et al. Ligand-induced conformational changes allosterically activate Toll-like receptor 9. Nat Immunol. 2007; 8:772–9.
Article25). Pålsson-McDermott EM, O'Neill LA. Building an immune system from nine domains. Biochem Soc Trans. 2007; 35:1437–44.
Article26). Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004; 16:3–9.
Article27). Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007; 13:460–9.28). Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003; 301:640–3.
Article29). Yamamoto M, Takeda K, Akira S. TIR domain-containing adaptors define the specificity of TLR signaling. Mol Immunol. 2004; 40:861–8.
Article30). Sato S, Sugiyama M, Yamamoto M, Watanabe Y, Kawai T, Takeda K, et al. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-kappa B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J Immunol. 2003; 171:4304–10.31). Johnson AC, Li X, Pearlman E. MyD88 functions as a negative regulator of TLR3/TRIF-induced corneal inflammation by inhibiting activation of c-Jun N-terminal kinase. J Biol Chem. 2008; 283:3988–96.
Article32). Sheedy FJ, O'Neill LA. The Troll in Toll: Mal and Tram as bridges for TLR2 and TLR4 signaling. J Leukoc Biol. 2007; 82:196–203.
Article33). Kenny EF, Talbot S, Gong M, Golenbock DT, Bryant CE, O'Neill LA. MyD88 adaptor-like is not essential for TLR2 signaling and inhibits signaling by TLR3. J Immunol. 2009; 183:3642–51.
Article34). Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001; 410:37–40.
Article35). Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006; 13:816–25.
Article36). Weiss DS, Raupach B, Takeda K, Akira S, Zychlinsky A. Toll-like receptors are temporally involved in host defense. J Immunol. 2004; 172:4463–9.
Article37). Campos MA, Closel M, Valente EP, Cardoso JE, Akira S, Alvarez-Leite JI, et al. Impaired production of proinflammatory cytokines and host resistance to acute infection with Trypanosoma cruzi in mice lacking functional myeloid differentiation factor 88. J Immunol. 2004; 172:1711–8.38). Lawley TD, Clare S, Walker AW, Goulding D, Stabler RA, Croucher N, et al. Antibiotic treatment of clostridium difficile carrier mice triggers a supershedder state, spore-mediated transmission, and severe disease in immunocompromised hosts. Infect Immun. 2009; 77:3661–9.39). Jarchum I, Liu M, Lipuma L, Pamer EG. Toll-like receptor 5 stimulation protects mice from acute clostridium difficile colitis. Infect Immun. 2011; 79:1498–503.40). Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol. 2000; 165:5392–6.41). Gerold G, Zychlinsky A, de Diego JL. What is the role of Toll-like receptors in bacterial infections? Semin Immunol. 2007; 19:41–7.
Article42). Miller LS, O'Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, et al. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006; 24:79–91.43). Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011; 34:637–50.
Article44). Andersen-Nissen E, Hawn TR, Smith KD, Nachman A, Lampano AE, Uematsu S, et al. Cutting edge: Tlr5–/–mice are more susceptible to Escherichia coli urinary tract infection. J Immunol. 2007; 178:4717–20.45). Feuillet V, Medjane S, Mondor I, Demaria O, Pagni PP, Galán JE, et al. Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc Natl Acad Sci U S A. 2006; 103:12487–92.
Article46). Uematsu S, Jang MH, Chevrier N, Guo Z, Kumagai Y, Yamamoto M, et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol. 2006; 7:868–74.
Article47). Arpaia N, Godec J, Lau L, Sivick KE, McLaughlin LM, Jones MB, et al. TLR signaling is required for Salmonella typhimurium virulence. Cell. 2011; 144:675–88.48). Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011; 30:16–34.
Article49). Ku CL, Yang K, Bustamante J, Puel A, von Bernuth H, Santos OF, et al. Inherited disorders of human Tolllike receptor signaling: immunological implications. Immunol Rev. 2005; 203:10–20.
Article50). Ku CL, von Bernuth H, Picard C, Zhang SY, Chang HH, Yang K, et al. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J Exp Med. 2007; 204:2407–22.
Article51). Casrouge A, Zhang SY, Eidenschenk C, Jouanguy E, Puel A, Yang K, et al. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science. 2006; 314:308–12.
Article52). Zhang SY, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007; 317:1522–7.
Article53). Guo Y, Audry M, Ciancanelli M, Alsina L, Azevedo J, Herman M, et al. Herpes simplex virus encephalitis in a patient with complete TLR3 deficiency: TLR3 is otherwise redundant in protective immunity. J Exp Med. 2011; 208:2083–98.
Article54). Pérez de Diego R, Sancho-Shimizu V, Lorenzo L, Puel A, Plancoulaine S, Picard C, et al. Human TRAF3 adaptor molecule deficiency leads to impaired Toll-like receptor 3 response and susceptibility to herpes simplex encephalitis. Immunity. 2010; 33:400–11.
Article55). Brinkmann MM, Spooner E, Hoebe K, Beutler B, Ploegh HL, Kim YM. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J Cell Biol. 2007; 177:265–75.
Article56). Iwami KI, Matsuguchi T, Masuda A, Kikuchi T, Musikacharoen T, Yoshikai Y. Cutting edge: naturally occurring soluble form of mouse Toll-like receptor 4 inhibits lipopolysaccharide signaling. J Immunol. 2000; 165:6682–6.
Article57). LeBouder E, Rey-Nores JE, Rushmere NK, Grigorov M, Lawn SD, Affolter M, et al. Soluble forms of Toll-like receptor (TLR)2 capable of modulating TLR2 signaling are present in human plasma and breast milk. J Immunol. 2003; 171:6680–9.
Article58). Brint EK, Xu D, Liu H, Dunne A, McKenzie AN, O'Neill LA, et al. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol. 2004; 5:373–9.
Article59). Garlanda C, Riva F, Polentarutti N, Buracchi C, Sironi M, De Bortoli M, et al. Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family. Proc Natl Acad Sci U S A. 2004; 101:3522–6.
Article60). Bergers G, Reikerstorfer A, Braselmann S, Graninger P, Busslinger M. Alternative promoter usage of the Fosresponsive gene Fit-1 generates mRNA isoforms coding for either secreted or membrane-bound proteins related to the IL-1 receptor. EMBO J. 1994; 13:1176–88.
Article61). Thomassen E, Renshaw BR, Sims JE. Identification and characterization of SIGIRR, a molecule representing a novel subtype of the IL-1R superfamily. Cytokine. 1999; 11:389–99.
Article62). Wald D, Qin J, Zhao Z, Qian Y, Naramura M, Tian L, et al. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2003; 4:920–7.
Article63). Janssens S, Burns K, Tschopp J, Beyaert R. Regulation of interleukin-1-and lipopolysaccharide-induced NF-kappaB activation by alternative splicing of MyD88. Curr Biol. 2002; 12:467–71.64). Janssens S, Beyaert R. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol Cell. 2003; 11:293–302.
Article65). Kobayashi K, Hernandez LD, Galán JE, Janeway CA Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002; 110:191–202.
Article66). Opipari AW Jr, Boguski MS, Dixit VM. The A20 cDNA induced by tumor necrosis factor alpha encodes a novel type of zinc finger protein. J Biol Chem. 1990; 265:14705–8.
Article67). Krikos A, Laherty CD, Dixit VM. Transcriptional activation of the tumor necrosis factor alpha-inducible zinc finger protein, A20, is mediated by kappa B elements. J Biol Chem. 1992; 267:17971–6.
Article68). Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004; 5:1052–60.
Article69). Seol W, Choi HS, Moore DD. An orphan nuclear hormone receptor that lacks a DNA binding domain and heterodimerizes with other receptors. Science. 1996; 272:1336–9.
Article70). Lee YS, Chanda D, Sim J, Park YY, Choi HS. Structure and function of the atypical orphan nuclear receptor small heterodimer partner. Int Rev Cytol. 2007; 261:117–58.
Article71). Yuk JM, Shin DM, Lee HM, Kim JJ, Kim SW, Jin HS, et al. The orphan nuclear receptor SHP acts as a negative regulator in inflammatory signaling triggered by Toll-like receptors. Nat Immunol. 2011; 12:742–51.
Article72). Leavy O. Innate immunity: SHP regulates TLR signalling. Nat Rev Immunol. 2011; 11:502.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neonatal innate immunity and Toll-like receptor
- The Innate Immune Responses in Pathogenesis of Chronic Rhinosinusitis
- The Gate Way of Communication between Microorganism and Human Body: Toll-like Receptors
- Innate Immunity and Organ Transplantation
- Activation of Innate Immune System During Viral Infection: Role of Pattern-recognition Receptors (PRRs) in Viral Infection