J Bacteriol Virol.
2008 Mar;38(1):29-37. 10.4167/jbv.2008.38.1.29.
Intracellular Localization of the Porcine Reproductive and Respiratory Syndrome Virus Nucleocapsid Protein
- Affiliations
-
- 1Department of Microbiology, College of Medicine and Medical Research Institute, Chungbuk National University, Cheongju, Korea. ymlee@chungbuk.ac.kr
- 2Research Institute of Veterinary Medicine, College of Veterinary Medicine, Chungbuk National University, Cheongju, Korea.
- KMID: 2168516
- DOI: http://doi.org/10.4167/jbv.2008.38.1.29
Abstract
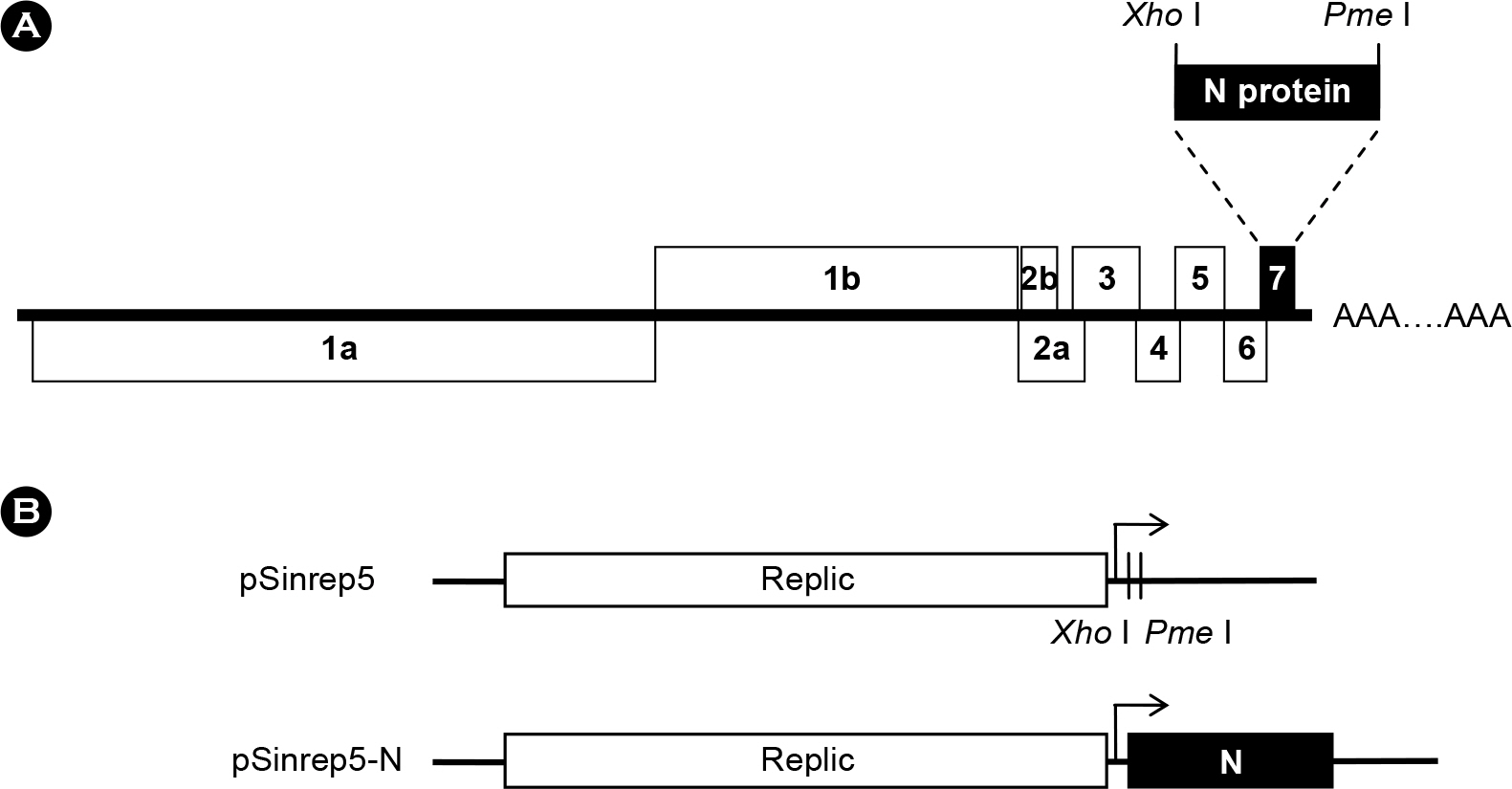
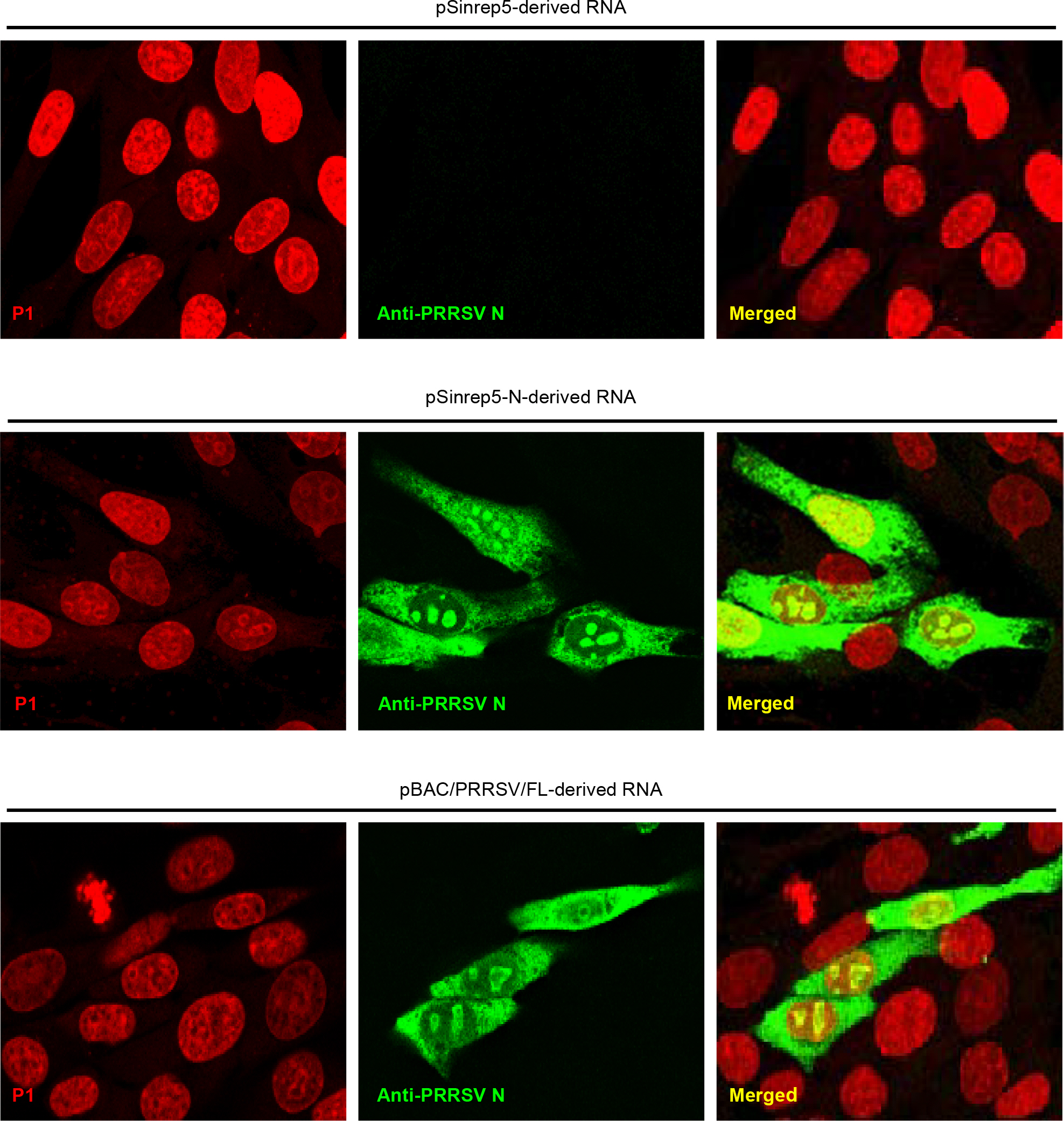
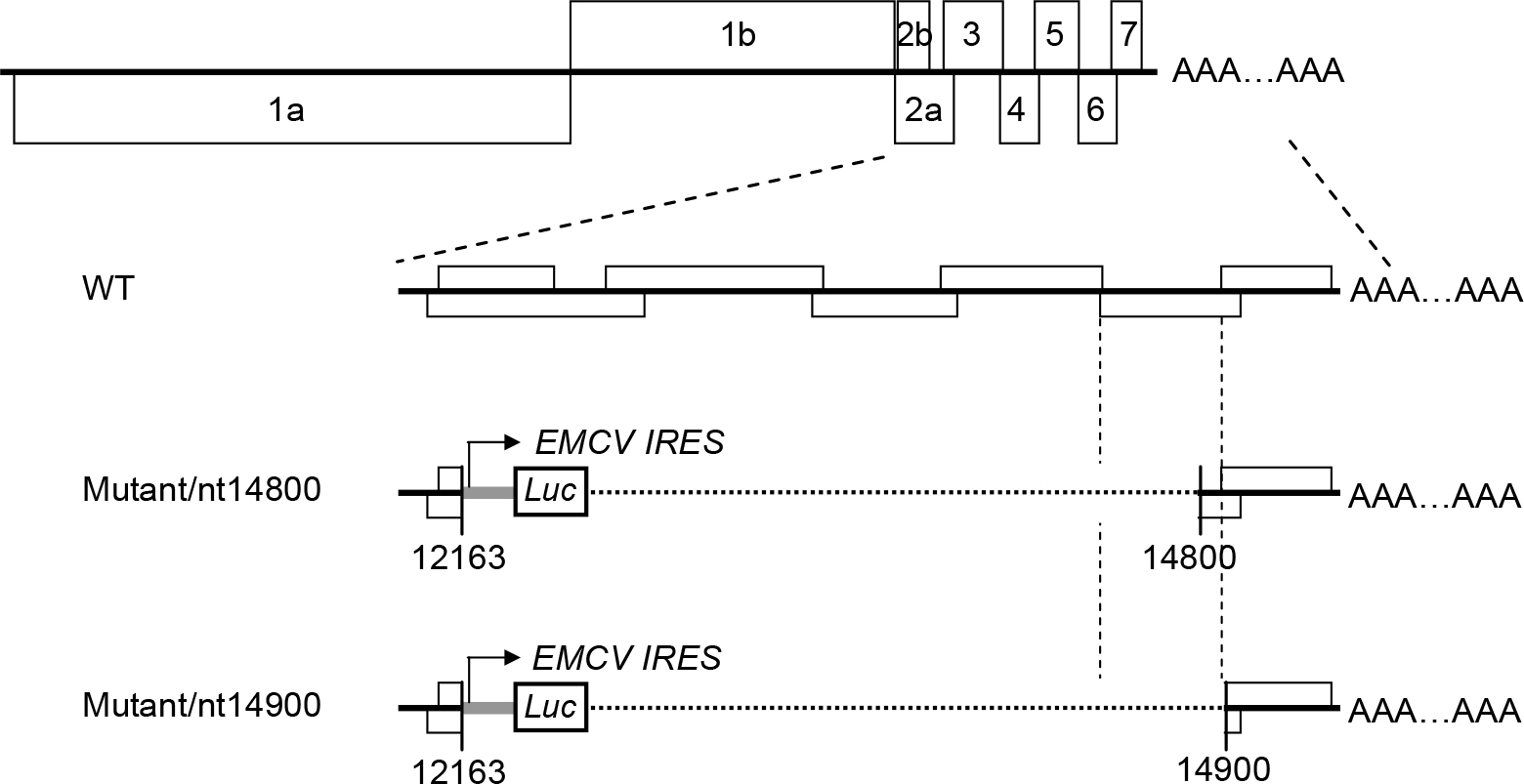
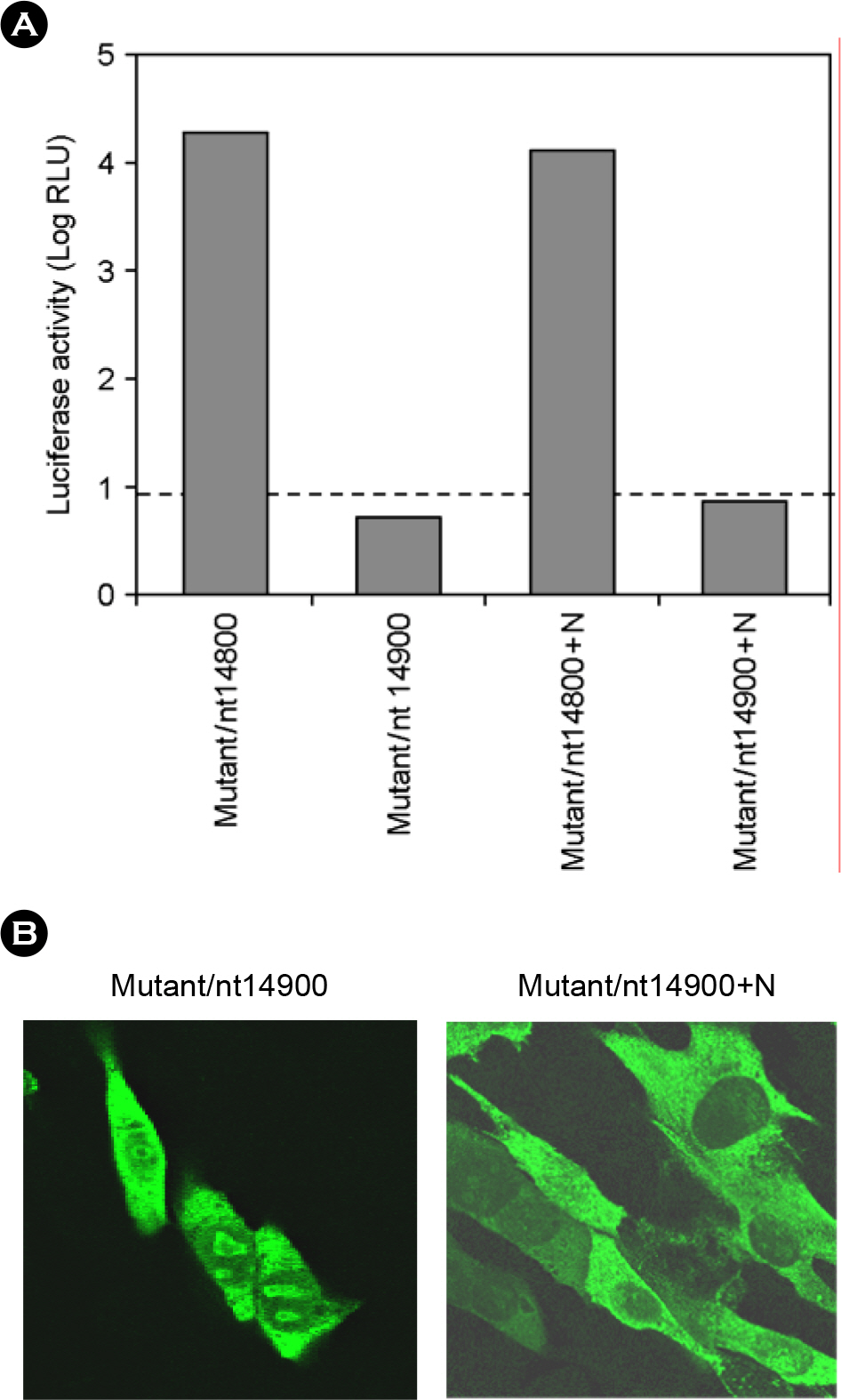
- Porcine reproductive and respiratory syndrome virus (PRRSV) is a small enveloped, positive-stranded RNA virus belonging to the family Arteriviridae. It causes the porcine reproductive and respiratory syndrome in swine. The virus has 7 structural proteins: Of the seven, the N protein is the nucleocapsid that comprises a core of the virus particle. We have expressed the N protein of PRRSV PL97-1/LP1 strain using a heterologous gene expression vector derived from Sindbis virus, called pSinrep5. Immunofluorescence analysis showed that the N proteins were mainly found in the cytoplasm as well as in the nucleus of BHK-21 cells transfected with pSinrep5-N-derived RNA. Moreover, expression of the N protein did not change the incompetence of RNA replication of Mutant/nt14900 that lacks a 3' cis-acting replication element and the efficiency of RNA replication of Mutant/nt14800 that has a low level of RNA replication. Overall, our findings are consistent with previous results and help to understand a role of the N protein in PRRSV biology.
Keyword
MeSH Terms
Figure
Reference
-
References
1). Agapov EV, Frolov I, Lindenbach BD, Pragai BM, Schlesinger S, Rice CM. Noncytopathic Sindbis virus RNA vectors for heterologous gene expression. Proc Natl Acad Sci USA. 95:12989–12994. 1998.
Article2). Almazan F, Galan C, Enjuanes L. The nucleoprotein is required for efficient coronavirus genome replication. J Virol. 78:12683–12688. 2004.
Article3). Aminev AG, Amineva SP, Palmenberg AC. Encephalomyocarditis virus (EMCV) proteins 2A and 3BCD localize to nuclei and inhibit cellular mRNA transcription but not rRNA transcription. Virus Res. 95:59–73. 2003.
Article4). Amineva SP, Aminev AG, Palmenberg AC, Gern JE. Rhinovirus 3C protease precursors 3CD and 3CD' localize to the nuclei of infected cells. J Gen Virol. 85:2969–2979. 2004.
Article5). Bautista EM, Faaberg KS, Mickelson D, McGruder ED. Functional properties of the predicted helicase of porcine reproductive and respiratory syndrome virus. Virology. 298:258–270. 2002.
Article6). Benfield DA, Nelson E, Collins JE, Harris L, Goyal SM, Robison D, Christianson WT, Morrison RB, Gorcyca D, Chladek D. Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332). J Vet Diagn Invest. 4:127–133. 1992.
Article7). Cavanagh D. Nidovirales: a new order comprising Corona-viridae and Arteriviridae. Arch Virol. 142:629–633. 1997.8). Chen H, Wurm T, Britton P, Brooks G, Hiscox JA. Interaction of the coronavirus nucleoprotein with nucleolar antigens and the host cell. J Virol. 76:5233–5250. 2002.
Article9). Choi YJ, Yun SI, Kang SY, Lee YM. Identification of 5′ and 3′ cis-acting elements of the porcine reproductive and respiratory syndrome virus: acquisition of novel 5′ AU-rich sequences restored replication of a 5′-proximal 7-nucleotide deletion mutant. J Virol. 80:723–736. 2006.10). Dea S, Gagnon CA, Mardassi H, Pirzadeh B, Rogan D. Current knowledge on the structural proteins of porcine reproductive and respiratory syndrome (PRRS) virus: comparison of the North American and European isolates. Arch Virol. 145:659–688. 2000.
Article11). Hiscox JA. The interaction of animal cytoplasmic RNA viruses with the nucleus to facilitate replication. Virus Res. 95:13–22. 2003.
Article12). Hiscox JA, Wurm T, Wilson L, Britton P, Cavanagh D, Brooks G. The coronavirus infectious bronchitis virus nucleoprotein localizes to the nucleolus. J Virol. 75:506–512. 2001.
Article13). Hoyt CC, Bouchard RJ, Tyler KL. Novel nuclear herniations induced by nuclear localization of a viral protein. J Virol. 78:6360–6369. 2004.
Article14). Kang SY, Choi YJ, Yun SI, Song BH, Lee YM. 5′ and 3′ cis-Acting RNA Elements Required for RNA Replication of Porcine Reproductive and Respiratory Syndrome Virus. J Bacteriol Virol. 37:193–201. 2007.15). Keffaber KK. Reproductive failure of unknown etiology. Am Assoc Swine Practitioners Newsl. 1:1–9. 1989.16). Lee C, Calvert JG, Welch SK, Yoo D. A DNA-launched reverse genetics system for porcine reproductive and respiratory syndrome virus reveals that homodimerization of the nucleocapsid protein is essential for virus infectivity. Virology. 331:47–62. 2005.
Article17). Meng XJ. Heterogeneity of porcine reproductive and respiratory syndrome virus: implications for current vaccine efficacy and future vaccine development. Vet Microbiol. 74:309–329. 2000.
Article18). Mng XJ, Paul PS, Halbur PG, Lum MA. Phylogenetic analysis of the putative M (ORF 6) and N (ORF 7) genes of porcine reproductive and respiratory syndrome virus (PRRSV): implication for the existence of two genotypes of PRRSV in the U.S.A. and Europe. Arch Virol. 140:745–755. 1995.19). Meulenberg JJ, Hulst MM, de Meijer EJ, Moonen PL, den Besten A, de Kluyver EP, Wensvoort G, Moormann RJ. Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology. 192:62–72. 1993.
Article20). Meulenberg JJ, Petersen-den Besten A, De Kluyver EP, Moormann RJ, Schaaper WM, Wensvoort G. Characterization of proteins encoded by ORFs 2 to 7 of Lelystad virus. Virology. 206:155–163. 1995.
Article21). Molenkamp R, van Tol H, Rozier BC, van der Meer Y, Spaan WJ, Snijder EJ. The arterivirus replicase is the only viral protein required for genome replication and subgenomic mRNA transcription. J Gen Virol. 81:2491–2496. 2000.
Article22). Nelsen CJ, Murtaugh MP, Faaberg KS. Porcine reproductive and respiratory syndrome virus comparison: divergent evolution on two continents. J Virol. 73:270–280. 1999.
Article23). Neumann EJ, Kliebenstein JB, Johnson CD, Mabry JW, Bush EJ, Seitzinger AH, Green AL, Zimmerman JJ. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J Am Vet Med Assoc. 227:385–392. 2005.
Article24). Rodriguez MJ, Sarraseca J, Garcia J, Sanz A, Plana-Duran J, Ignacio Casal J. Epitope mapping of the nucleocapsid protein of European and North American isolates of porcine reproductive and respiratory syndrome virus. J Gen Virol. 78:2269–2278. 1997.
Article25). Rowland RR, Kervin R, Kuckleburg C, Sperlich A, Benfield DA. The localization of porcine reproductive and respiratory syndrome virus nucleocapsid protein to the nucleolus of infected cells and identification of a potential nucleolar localization signal sequence. Virus Res. 64:1–12. 1999.
Article26). Rowland RR, Schneider P, Fang Y, Wootton S, Yoo D, Benfield DA. Peptide domains involved in the localization of the porcine reproductive and respiratory syndrome virus nucleocapsid protein to the nucleolus. Virology. 316:135–145. 2003.
Article27). Rowland RR, Yoo D. Nucleolar-cytoplasmic shuttling of PRRSV nucleocapsid protein: a simple case of molecular mimicry or the complex regulation by nuclear import, nucleolar localization and nuclear export signal sequences. Virus Res. 95:23–33. 2003.
Article28). Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed.Cold Spring Harbor Laboratory;Cold Spring Harbor, NY: 1989.29). Schelle B, Karl N, Ludewig B, Siddell SG, Thiel V. Selective replication of coronavirus genomes that express nucleocapsid protein. J Virol. 79:6620–6630. 2005.
Article30). Snijder EJ, Meulenberg JJ. The molecular biology of arteri-viruses. J Gen Virol. 79:961–979. 1998.
Article31). Thiel V, Karl N, Schelle B, Disterer P, Klagge I, Siddell SG. Multigene RNA vector based on coronavirus transcription. J Virol. 77:9790–9798. 2003.
Article32). van Dinten LC, Rensen S, Gorbalenya AE, Snijder EJ. Proteolytic processing of the open reading frame 1b-encoded part of arterivirus replicase is mediated by nsp4 serine protease and is essential for virus replication. J Virol. 73:2027–2037. 1999.
Article33). Wensvoort G, Tepstra C, Pol JM, ter Laak EA, Bloemraad M, de Kluyver EP, Kragten C, van Buiten L, den Besten A, Wagenaar F, Broekhuijsen JM, Moonen PLJM, Zestra T, de Boer EA, Tibben HJ, de Jong MF, van Veld P, Groenland GJR, van Gennep JA, Voets MT, Verheijden JHM, Braamskamp J. Mystery swine disease in The Netherlands: the isolation of Lelystad virus. Vet Q. 13:121–130. 1991.
Article34). Wieringa R, de Vries AA, van der Meulen J, Godeke GJ, Onderwater JJ, van Tol H, Koerten HK, Mommaas AM, Snijder EJ, Rottier PJ. Structural protein requirements in equine arteritis virus assembly. J Virol. 78:13019–13027. 2004.
Article35). Wootton S, Yoo D, Rogan D. Full-length sequence of a Canadian porcine reproductive and respiratory syndrome virus (PRRSV) isolate. Arch Virol. 145:2297–2323. 2000.
Article36). Wootton SK, Nelson EA, Yoo D. Antigenic structure of the nucleocapsid protein of porcine reproductive and respiratory syndrome virus. Clin Diagn Lab Immunol. 5:773–779. 1998.
Article37). Wootton SK, Rowland RR, Yoo D. Phosphorylation of the porcine reproductive and respiratory syndrome virus (PRRSV) nucleocapsid protein. J Virol. 76:10569–10576. 2002.38). Wu WH, Fang Y, Farwell R, Steffen-Bien M, Rowland RR, Christopher-Hennings J, Nelson EA. A 10-kDa structural protein of porcine reproductive and respiratory syndrome virus encoded by ORF2b. Virology. 287:183–191. 2001.
Article39). Wurm T, Chen H, Hodgson T, Britton P, Brooks G, Hiscox JA. Localization to the nucleolus is a common feature of coronavirus nucleoproteins, and the protein may disrupt host cell division. J Virol. 75:9345–9356. 2001.
Article40). Yoo D, Wootton SK, Li G, Song C, Rowland RR. Colocalization and interaction of the porcine arterivirus nucleocapsid protein with the small nucleolar RNA-associated protein fibrillarin. J Virol. 77:12173–12183. 2003.
Article41). Yun SI, Kim SY, Rice CM, Lee YM. Development and application of a reverse genetics system for Japanese encephalitis virus. J Virol. 77:6450–6465. 2003.
Article42). Zevenhoven-Dobbe JC, Greve S, van Tol H, Spaan WJ, Snijder EJ. Rescue of disabled infectious single-cycle (DISC) equine arteritis virus by using complementing cell lines that express minor structural glycoproteins. J Gen Virol. 85:3709–3714. 2004.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development and characterization of stable cell lines constitutively expressing the porcine reproductive and respiratory syndrome virus nucleocapsid protein
- Expression, Purification and Antiserum Production of the Porcine Reproductive and Respiratory Syndrome Virus Nsp1a Protein
- The signal sequence of type II porcine reproductive and respiratory syndrome virus glycoprotein 3 is sufficient for endoplasmic reticulum retention
- Porcine reproductive and respiratory syndrome virus vaccine does not fit in classical vaccinology
- Porcine ear necrosis syndrome by coinfection of porcine reproductive and respiratory syndrome virus and Staphylococcus hyicus