J Vet Sci.
2013 Sep;14(3):307-313. 10.4142/jvs.2013.14.3.307.
The signal sequence of type II porcine reproductive and respiratory syndrome virus glycoprotein 3 is sufficient for endoplasmic reticulum retention
- Affiliations
-
- 1Department of Infectious Disease, College of Veterinary Medicine, Konkuk University, Seoul 143-701, Korea.
- 2Animal and Plant Quarantine Agency, Anyang 430-757, Korea. slee0103@korea.kr
- KMID: 1705561
- DOI: http://doi.org/10.4142/jvs.2013.14.3.307
Abstract
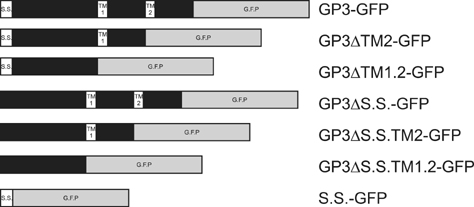
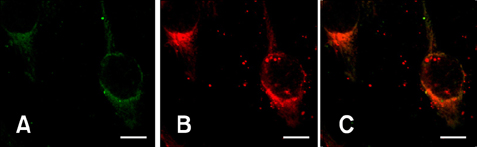
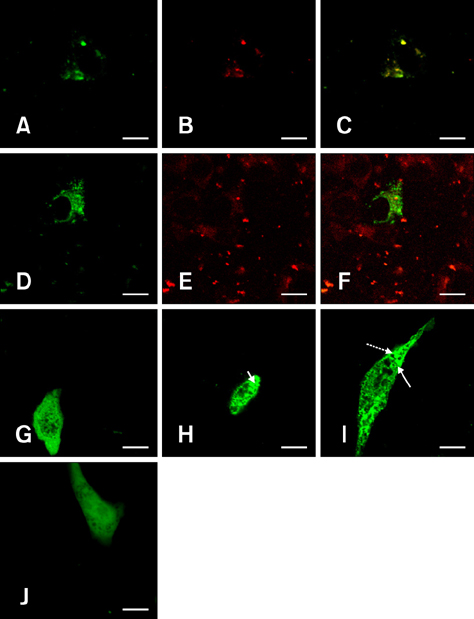
- The glycoprotein 3 (GP3) of type II porcine reproductive and respiratory syndrome virus has the characteristic domains of a membrane protein. However, this protein has been reported to be retained in the endoplasmic reticulum (ER) rather than transported to the plasma membrane of the cell. In this study, we performed confocal laser scanning microscopy analysis of variants of GP3 and foundthat the signal sequence of the GP3 led to confinement of GP3 in the ER, while the functional ortransmembrane domain did not affect its localization. Based on these results, we concludedthat the signal sequence of GP3 contains the ER retention signal, which might play an important role in assembly of viral proteins.
Keyword
MeSH Terms
-
Animals
Cell Line
Cell Membrane/*metabolism/virology
Cricetinae
Endoplasmic Reticulum/*metabolism/virology
Microscopy, Confocal/veterinary
Plasmids/genetics/metabolism
Porcine respiratory and reproductive syndrome virus/*genetics/metabolism
*Protein Sorting Signals
Sequence Analysis, Protein/veterinary
Viral Envelope Proteins/chemistry/*genetics/metabolism
Protein Sorting Signals
Viral Envelope Proteins
Figure
Reference
-
1. Ahn K, Szczesna-Skorupa E, Kemper B. The amino-terminal 29 amino acids of cytochrome P450 2C1 are sufficient for retention in the endoplasmic reticulum. J Biol Chem. 1993; 268:18726–18733.
Article2. Ansari IH, Kwon B, Osorio FA, Pattnaik AK. Influence of N-linked glycosylation of porcine reproductive and respiratory syndrome virus GP5 on virus infectivity, antigenicity, and ability to induce neutralizing antibodies. J Virol. 2006; 80:3994–4004.
Article3. Ashiru O, Bennett NJ, Boyle LH, Thomas M, Trowsdale J, Wills MR. NKG2D ligand MICA is retained in the cis-Golgi apparatus by human cytomegalovirus protein UL142. J Virol. 2009; 83:12345–12354.
Article4. Blobel G, Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975; 67:835–851.
Article5. Blobel G, Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975; 67:852–862.
Article6. Calvert JG, Slade DE, Shields SL, Jolie R, Mannan RM, Ankenbauer RG, Welch SK. CD163 expression confers susceptibility to porcine reproductive and respiratory syndrome viruses. J Virol. 2007; 81:7371–7379.
Article7. Cocquerel L, Duvet S, Meunier JC, Pillez A, Cacan R, Wychowski C, Dubuisson J. The transmembrane domain of hepatitis C virus glycoprotein E1 is a signal for static retention in the endoplasmic reticulum. J Virol. 1999; 73:2641–2649.
Article8. Cocquerel L, Meunier JC, Pillez A, Wychowski C, Dubuisson J. A retention signal necessary and sufficient for endoplasmic reticulum localization maps to the transmembrane domain of hepatitis C virus glycoprotein E2. J Virol. 1998; 72:2183–2191.
Article9. Collins JE, Benfield DA, Christianson WT, Harris L, Hennings JC, Shaw DP, Goyal SM, McCullough S, Morrison RB, Joo HS. Isolation of swine infertility and respiratory syndrome virus (isolate ATCC VR-2332) in North America and experimental reproduction of the disease in gnotobiotic pigs. J Vet Diagn Invest. 1992; 4:117–126.
Article10. Cserzö M, Wallin E, Simon I, von Heijne G, Elofsson A. Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Eng. 1997; 10:673–676.
Article11. Das PB, Dinh PX, Ansari IH, de Lima M, Osorio FA, Pattnaik AK. The minor envelope glycoproteins GP2a and GP4 of porcine reproductive and respiratory syndrome virus interact with the receptor CD163. J Virol. 2010; 84:1731–1740.
Article12. de Lima M, Ansari IH, Das PB, Ku BJ, Martinez-Lobo FJ, Pattnaik AK, Osorio FA. GP3 is a structural component of the PRRSV type II (US) virion. Virology. 2009; 390:31–36.
Article13. Gagnon CA, Lachapelle G, Langelier Y, Massie B, Dea S. Adenoviral-expressed GP5 of porcine respiratory and reproductive syndrome virus differs in its cellular maturation from the authentic viral protein but maintains known biological functions. Arch Virol. 2003; 148:951–972.
Article14. Gonin P, Mardassi H, Gagnon CA, Massie B, Dea S. A nonstructural and antigenic glycoprotein is encoded by ORF3 of the IAF-Klop strain of porcine reproductive and respiratory syndrome virus. Arch Virol. 1998; 143:1927–1940.
Article15. Hedges JF, Balasuriya UB, MacLachlan NJ. The open reading frame 3 of equine arteritis virus encodes an immunogenic glycosylated, integral membrane protein. Virology. 1999; 264:92–98.
Article16. Jiang W, Jiang P, Li Y, Wang X, Du Y. Analysis of immunogenicity of minor envelope protein GP3 of porcine reproductive and respiratory syndrome virus in mice. Virus Genes. 2007; 35:695–704.
Article17. Kanner EM, Klein IK, Friedlander M, Simon SM. The amino terminus of opsin translocates "posttranslationally" as efficiently as cotranslationally. Biochemistry. 2002; 41:7707–7715.
Article18. Katz JB, Shafer AL, Eernisse KA, Landgraf JG, Nelson EA. Antigenic differences between European and American isolates of porcine reproductive and respiratory syndrome virus (PRRSV) are encoded by the carboxyterminal portion of viral open reading frame 3. Vet Microbiol. 1995; 44:65–76.
Article19. Keffaber KK. Reproductive failure of unknown etiology. Am Assoc Swine Pract Newsl. 1989; 1:1–9.20. Krogerus C, Samuilova O, Pöyry T, Jokitalo E, Hyypiä T. Intracellular localization and effects of individually expressed human parechovirus 1 non-structural proteins. J Gen Virol. 2007; 88(Pt 3):831–841.
Article21. Lingappa VR, Katz FN, Lodish HF, Blobel G. A signal sequence for the insertion of a transmembrane glycoprotein. Similarities to the signals of secretory proteins in primary structure and function. J Biol Chem. 1978; 253:8667–8670.
Article22. Mardassi H, Gonin P, Gagnon CA, Massie B, Dea S. A subset of porcine reproductive and respiratory syndrome virus GP3 glycoprotein is released into the culture medium of cells as a non-virion-associated and membrane-free (soluble) form. J Virol. 1998; 72:6298–6306.
Article23. Mardassi H, Massie B, Dea S. Intracellular synthesis, processing, and transport of proteins encoded by ORFs 5 to 7 of porcine reproductive and respiratory syndrome virus. Virology. 1996; 221:98–112.
Article24. Mateu E, Diaz I. The challenge of PRRS immunology. Vet J. 2008; 177:345–351.
Article25. Meng XJ, Paul PS, Halbur PG, Morozov I. Sequence comparison of open reading frames 2 to 5 of low and high virulence United States isolates of porcine reproductive and respiratory syndrome virus. J Gen Virol. 1995; 76(Pt 12):3181–3188.
Article26. Meulenberg JJ, Petersen-den Besten A. Identification and characterization of a sixth structural protein of Lelystad virus: the glycoprotein GP2 encoded by ORF2 is incorporated in virus particles. Virology. 1996; 225:44–51.
Article27. Meulenberg JJM, Petersen-den Besten A, De Kluyver EP, Moormann RJM, Schaaper WM, Wensvoort G. Characterization of proteins encoded by ORFs 2 to 7 of Lelystad virus. Virology. 1995; 206:155–163.
Article28. Meulenberg JJ, van Nieuwstadt AP, van Essen-Zandbergen A, Langeveld JP. Posttranslational processing and identification of a neutralization domain of the GP4 protein encoded by ORF4 of Lelystad virus. J Virol. 1997; 71:6061–6067.
Article29. Molenkamp R, van Tol H, Rozier BC, van der Meer Y, Spaan WJ, Snijder EJ. The arterivirus replicase is the only viral protein required for genome replication and subgenomic mRNA transcription. J Gen Virol. 2000; 81:2491–2496.
Article30. Ostrowski M, Galeota JA, Jar AM, Platt KB, Osorio FA, Lopez OJ. Identification of neutralizing and nonneutralizing epitopes in the porcine reproductive and respiratory syndrome virus GP5 ectodomain. J Virol. 2002; 76:4241–4250.
Article31. Pedrazzini E, Villa A, Borgese N. A mutant cytochrome b5 with a lengthened membrane anchor escapes from the endoplasmic reticulum and reaches the plasma membrane. Proc Natl Acad Sci U S A. 1996; 93:4207–4212.
Article32. Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011; 8:785–786.
Article33. Snijder EJ, van Tol H, Roos N, Pedersen KW. Non-structural proteins 2 and 3 interact to modify host cell membranes during the formation of the arterivirus replication complex. J Gen Virol. 2001; 82:985–994.
Article34. Stornaiuolo M, Lotti LV, Borgese N, Torrisi MR, Mottola G, Martire G, Bonatti S. KDEL and KKXX retrieval signals appended to the same reporter protein determine different trafficking between endoplasmic reticulum, intermediate compartment, and Golgi complex. Mol Biol Cell. 2003; 14:889–902.
Article35. Van Gorp H, Van Breedam W, Delputte PL, Nauwynck HJ. Sialoadhesin and CD163 join forces during entry of the porcine reproductive and respiratory syndrome virus. J Gen Virol. 2008; 89:2943–2953.
Article36. Wensvoort G, Terpstra C, Pol JMA, ter Laak EA, Bloemraad M, de Kluyver EP, Kragten C, van Buiten L, den Besten A, Wagenaar F, Broekhuijsen JM, Moonen PLJM, Zetstra T, de Boer EA, Tibben HJ, de Jong MF, van't Veld P, Groenland GJR, van Gennep JA, Voets MT, Verheijden JHM, Braamskamp J. Mystery swine disease in The Netherlands: the isolation of Lelystad virus. Vet Q. 1991; 13:121–130.
Article37. Wieringa R, de Vries AAF, Raamsman MJB, Rottier PJM. Characterization of two new structural glycoproteins, GP3 and GP4, of equine arteritis virus. J Virol. 2002; 76:10829–10840.
Article38. Wissink EH, Kroese MV, van Wijk HA, Rijsewijk FA, Meulenberg JJ, Rottier PJ. Envelope protein requirements for the assembly of infectious virions of porcine reproductive and respiratory syndrome virus. J Virol. 2005; 79:12495–12506.
Article39. Yu M, Liu X, Sun L, Chen C, Ma G, Kitamura Y, Gao GF, Liu W. Subcellular localization and topology of porcine reproductive and respiratory syndrome virus E protein. Virus Res. 2010; 152:104–114.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevalence of porcine reproductive and respiratory syndrome virus, porcine circovirus type 2 and porcine parvovirus from aborted fetuses and pigs with respiratory problems in Korea
- Phylogenetic characterization of genes encoding for glycoprotein 5 and membrane protein of PRRSV isolate HH08
- Augmented immune responses in pigs immunized with an inactivated porcine reproductive and respiratory syndrome virus containing the deglycosylated glycoprotein 5 under field conditions
- A survey of porcine reproductive and respiratory syndrome among wild boar populations in Korea
- Genetic diversity of porcine reproductive and respiratory syndrome virus in Korea