Hanyang Med Rev.
2013 May;33(2):123-129. 10.7599/hmr.2013.33.2.123.
Amyloidogenic Protein of alpha-Synuclein
- Affiliations
-
- 1School of Chemical and Biological Engineering, Seoul National University, Seoul, Korea. srpaik@snu.ac.kr
- KMID: 2168245
- DOI: http://doi.org/10.7599/hmr.2013.33.2.123
Abstract
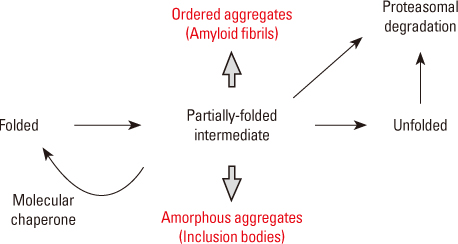
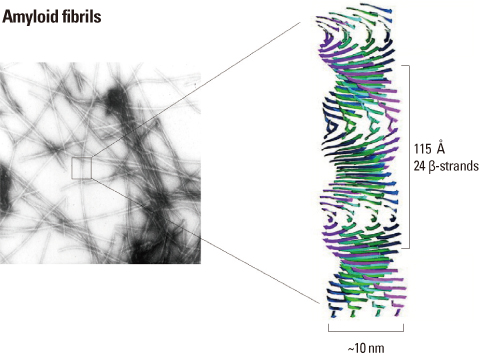
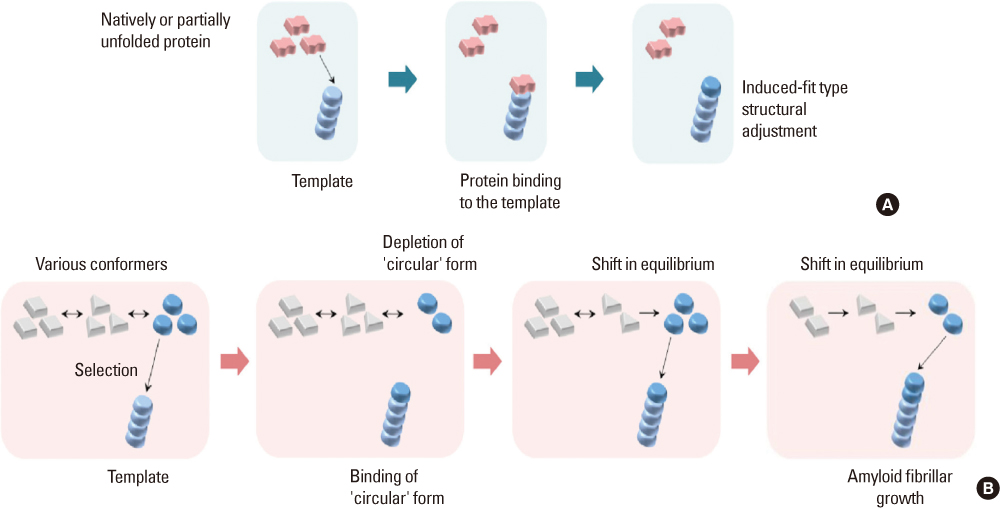
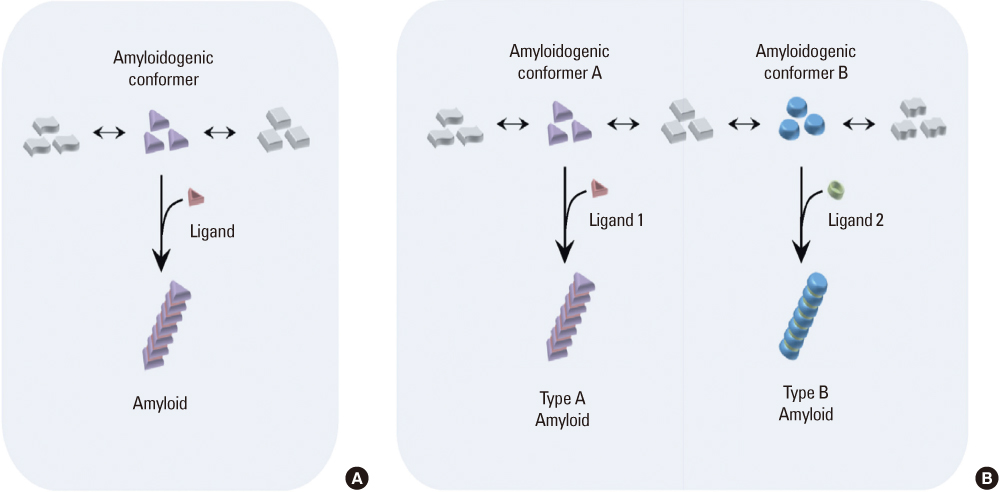
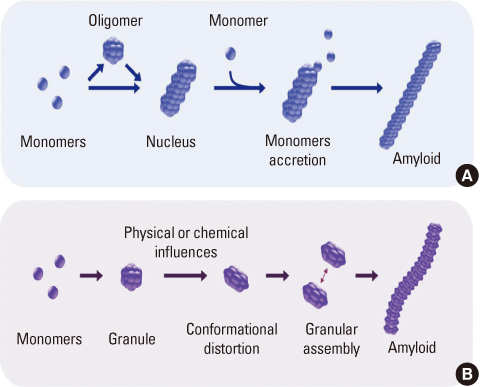
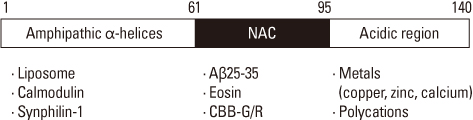
- Amyloidogenesis is the key pathological phenomenon commonly observed in various neurodegenerative disorders. alpha-Synuclein is the major constituent of Lewy bodies as a common pathological signature of Lewy body diseases (LBDs) including Parkinson's disease (PD), PD with dementia (PDD), and Dementia with Lewy bodies (DLB). As proteins unfold, they would result in producing either ordered or disordered aggregates unless they are folded back to the native state by molecular chaperones or removed via proteolytic degradation. alpha-Synuclein known as a natively unfolded protein has self-assembled into the ordered protein aggregates of amyloid fibrils which comprise the radiating filaments found in Lewy bodies. Amyloid fibrils are generated via either a template-dependent or template-independent mechanism. The prevalent nucleation-dependent fibrillation accelerates the assembly process in the presence of seeds such as prefibrillar species. As a template-independent process, we have recently proposed the double-concerted fibrillation mechanism in which the oligomeric species of alpha-synuclein act as a growing unit to form the mature fibrils. Despite insufficient understanding of the toxic causes of alpha-synuclein, the oligomeric species have been suggested to be responsible for the cellular degeneration by influencing membrane stability while leaving the amyloid fibrils as a detoxification end product. Alternatively, the transition process from the oligomers to the fibrils has been proposed to affect cell viability. It is, therefore, expected to develop prophylactic and therapeutic strategies toward the synucleinopathies by studying cellular function of alpha-synuclein, molecular mechanism of its assembly into the amyloid fibrils, and their effects on cellular biogenesis. By studying cellular function of alpha-synuclein, its molecular mechanism of assembly into amyloid fibrils and their effects on cellular biogenesis, progress of prophylactic and therapeutic strategies toward synucleinopathy can be seen.
MeSH Terms
Figure
Cited by 1 articles
-
Do Reactive Oxygen Species Cause Aging?
Seong Eon Ryu
Hanyang Med Rev. 2013;33(2):75-76. doi: 10.7599/hmr.2013.33.2.75.
Reference
-
1. Sipe JD. Amyloidosis. Annu Rev Biochem. 1992; 61:947–975.
Article2. Virchow R. Zur cellulose-frage. Virchows Archiv. 1855; 8:140–144.
Article3. Paik SR, Lee JH. Strategy to control the protein aggregation: neurodegenerative disorders and amyloid. Biochem Mol Biol News. 2007; 27:23–29.4. Braak H, Braak E. Pathoanatomy of Parkinson's disease. J Neurol. 2000; 247:Suppl 2. II3–II10.
Article5. Shults CW. Lewy bodies. Proc Natl Acad Sci U S A. 2006; 103:1661–1668.
Article6. Sunde M, Blake CC. From the globular to the fibrous state: protein structure and structural conversion in amyloid formation. Q Rev Biophys. 1998; 31:1–39.
Article7. Knowles TP, Fitzpatrick AW, Meehan S, Mott HR, Vendruscolo M, Dobson CM, et al. Role of intermolecular forces in defining material properties of protein nanofibrils. Science. 2007; 318:1900–1903.
Article8. Tan SY, Pepys MB. Amyloidosis. Histopathology. 1994; 25:403–414.
Article9. Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003; 349:583–596.
Article10. Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT Jr. Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002; 418:291.11. Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003; 26:267–298.
Article12. Cuajungco MP, Goldstein LE, Nunomura A, Smith MA, Lim JT, Atwood CS, et al. Evidence that the beta-amyloid plaques of Alzheimer's disease represent the redox-silencing and entombment of abeta by zinc. J Biol Chem. 2000; 275:19439–19442.
Article13. Opazo C, Huang X, Cherny RA, Moir RD, Roher AE, White AR, et al. Metalloenzyme-like activity of Alzheimer's disease beta-amyloid. Cu-dependent catalytic conversion of dopamine, cholesterol, and biological reducing agents to neurotoxic H(2)O(2). J Biol Chem. 2002; 277:40302–40308.14. Bhak G, Choe YJ, Paik SR. Mechanism of amyloidogenesis: nucleation-dependent fibrillation versus double-concerted fibrillation. BMB Rep. 2009; 42:541–551.
Article15. Naiki H, Gejyo F. Kinetic analysis of amyloid fibril formation. Methods Enzymol. 1999; 309:305–318.16. Jarrett JT, Lansbury PT Jr. Seeding "one-dimensional crystallization" of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie? Cell. 1993; 73:1055–1058.
Article17. Harper JD, Lansbury PT Jr. Models of amyloid seeding in Alzheimer's disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu Rev Biochem. 1997; 66:385–407.
Article18. Wood SJ, Wypych J, Steavenson S, Louis JC, Citron M, Biere AL. alpha-synuclein fibrillogenesis is nucleation-dependent. Implications for the pathogenesis of Parkinson's disease. J Biol Chem. 1999; 274:19509–19512.19. Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997; 276:2045–2047.
Article20. Ulmer TS, Bax A, Cole NB, Nussbaum RL. Structure and dynamics of micelle-bound human alpha-synuclein. J Biol Chem. 2005; 280:9595–9603.21. Eliezer D, Kutluay E, Bussell R Jr, Browne G. Conformational properties of alpha-synuclein in its free and lipid-associated states. J Mol Biol. 2001; 307:1061–1073.
Article22. Recchia A, Debetto P, Negro A, Guidolin D, Skaper SD, Giusti P. Alpha-synuclein and Parkinson's disease. FASEB J. 2004; 18:617–626.23. Giasson BI, Murray IV, Trojanowski JQ, Lee VM. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J Biol Chem. 2001; 276:2380–2386.
Article24. Iwai A, Masliah E, Yoshimoto M, Ge N, Flanagan L, de Silva HA, et al. The precursor protein of non-A beta component of Alzheimer's disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995; 14:467–475.
Article25. Withers GS, George JM, Banker GA, Clayton DF. Delayed localization of synelfin (synuclein, NACP) to presynaptic terminals in cultured rat hippocampal neurons. Brain Res Dev Brain Res. 1997; 99:87–94.
Article26. Jakes R, Spillantini MG, Goedert M. Identification of two distinct synucleins from human brain. FEBS Lett. 1994; 345:27–32.
Article27. Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, et al. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J Neurosci. 2002; 22:8797–8807.
Article28. Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000; 25:239–252.
Article29. Scott DA, Tabarean I, Tang Y, Cartier A, Masliah E, Roy S. A pathologic cascade leading to synaptic dysfunction in alpha-synuclein-induced neurodegeneration. J Neurosci. 2010; 30:8083–8095.
Article30. Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, et al. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010; 65:66–79.
Article31. Lundblad M, Decressac M, Mattsson B, Bjorklund A. Impaired neurotransmission caused by overexpression of alpha-synuclein in nigral dopamine neurons. Proc Natl Acad Sci U S A. 2012; 109:3213–3219.
Article32. Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010; 329:1663–1667.33. Vekrellis K, Xilouri M, Emmanouilidou E, Rideout HJ, Stefanis L. Pathological roles of alpha-synuclein in neurological disorders. Lancet Neurol. 2011; 10:1015–1025.34. Iwata A, Maruyama M, Akagi T, Hashikawa T, Kanazawa I, Tsuji S, et al. Alpha-synuclein degradation by serine protease neurosin: implication for pathogenesis of synucleinopathies. Hum Mol Genet. 2003; 12:2625–2635.
Article35. Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004; 305:1292–1295.
Article36. Kragh CL, Ubhi K, Wyss-Coray T, Masliah E. Autophagy in dementias. Brain Pathol. 2012; 22:99–109.
Article37. Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998; 4:1318–1320.
Article38. Kosaka K. Diffuse Lewy body disease in Japan. J Neurol. 1990; 237:197–204.
Article39. Colla E, Jensen PH, Pletnikova O, Troncoso JC, Glabe C, Lee MK. Accumulation of toxic alpha-synuclein oligomer within endoplasmic reticulum occurs in alpha-synucleinopathy in vivo. J Neurosci. 2012; 32:3301–3305.
Article40. Horvath I, Weise CF, Andersson EK, Chorell E, Sellstedt M, Bengtsson C, et al. Mechanisms of protein oligomerization: inhibitor of functional amyloids templates alpha-synuclein fibrillation. J Am Chem Soc. 2012; 134:3439–3444.
Article41. Iwatsubo T. Pathological biochemistry of alpha-synucleinopathy. Neuropathology. 2007; 27:474–478.42. Souza JM, Giasson BI, Chen Q, Lee VM, Ischiropoulos H. Dityrosine cross-linking promotes formation of stable alpha -synuclein polymers. Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J Biol Chem. 2000; 275:18344–18349.
Article43. Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson's disease and heavy metal exposure. J Biol Chem. 2001; 276:44284–44296.
Article44. Conway KA, Rochet JC, Bieganski RM, Lansbury PT Jr. Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001; 294:1346–1349.
Article45. Danzer KM, Haasen D, Karow AR, Moussaud S, Habeck M, Giese A, et al. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci. 2007; 27:9220–9232.
Article46. Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of alpha-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013; 14:38–48.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Protein Transmission, Seeding and Degradation: Key Steps for alpha-Synuclein Prion-Like Propagation
- Alpha-Synuclein Function and Dysfunction on Cellular Membranes
- Accumulation of Alpha-synuclein Causes Colonic Dysmotility Independently of Enteric Nervous Damage in the Early Stage of Parkinson's Disease (Neurogastroenterol Motil 2012;24:e425-e436)
- Topographical Propagation of alpha-synuclein Pathology in Parkinson's Disease: Phenomenology and Hypothetical Mechanism
- Structure, Distribution, and Genetic Profile of α-Synuclein and Their Potential Clinical Application in Parkinson's Disease