Clin Nutr Res.
2016 Jan;5(1):33-42. 10.7762/cnr.2016.5.1.33.
Fasting Glucose is a Useful Indicator for Cerebrovascular Risk in Non-Diabetic Koreans: Association With Oxidative Stress and Inflammation
- Affiliations
-
- 1Department of Food Science Nutrition, Brain Busan 21 project, Dong-A University, Busan, 49315 Korea. oykim@dau.ac.kr
- 2Department of Neurology, Busan-Ulsan Regional Cardiocerebrovascular Center, Dong-A University Hospital, College of Medicine, Busan, 49201 Korea.
- KMID: 2162593
- DOI: http://doi.org/10.7762/cnr.2016.5.1.33
Abstract
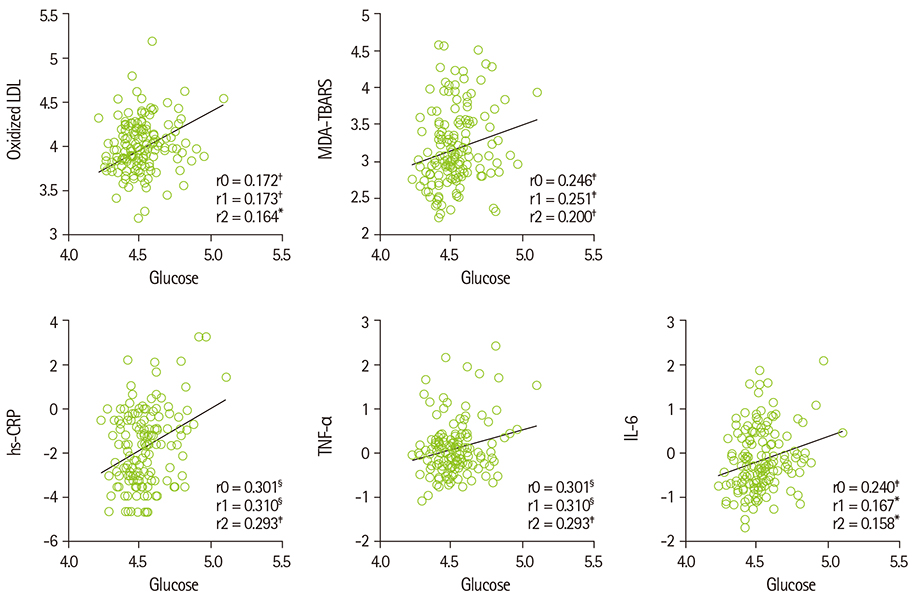
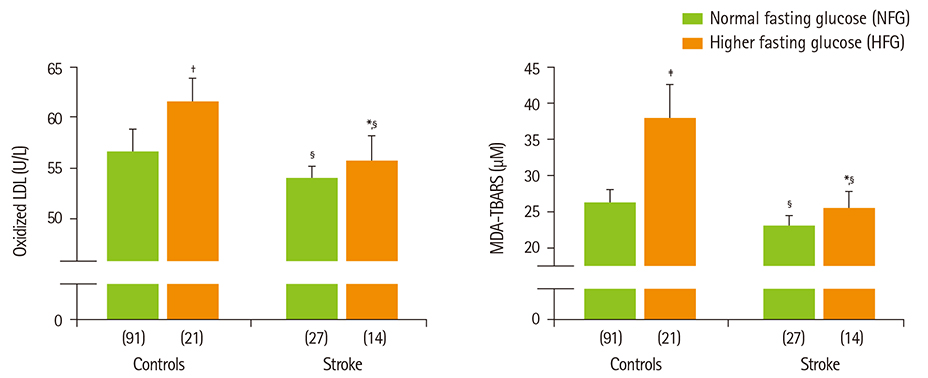
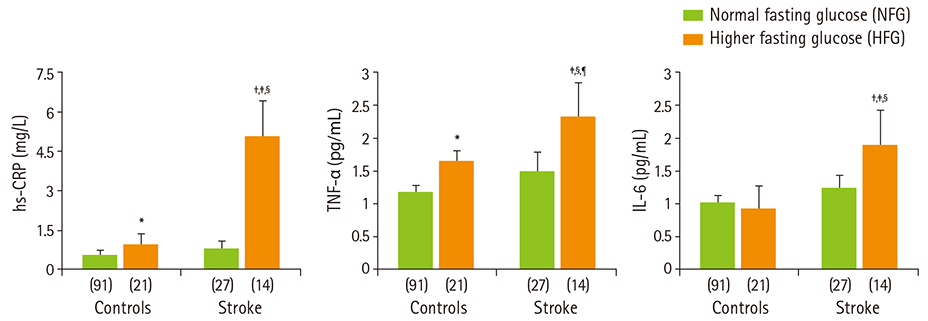
- Diabetes and impaired fasting glucose are associated with incidence of cerebro-/cardio-vascular diseases. This study hypothesized that fasting glycemic status may reflect cerebrovascular risk in non-diabetic Koreans. Fasting glycemic status, lipid profiles, oxidative stress, and inflammation markers were measured in non-diabetic subjects (healthy controls, n = 112 and stroke n = 41). Systolic blood pressure, fasting glucose, glycated hemoglobin (HbA1C), triglycerides, high sensitivity C-reactive protein (hs-CPR), interleukin-6, and tumor necrosis factor-alpha were higher, and high density lipoprotein (HDL)-cholesterols were lower in patients with stroke than healthy controls. Fasting glucose positively correlated with hs-CRP, interleukin-6, tumor necrosis factor-alpha, oxidized low density lipoprotein (LDL) and malondialdehyde. The significances continued or at least turned to a trend after adjustments for confounding factors. Multiple regression analyses revealed that fasting glucose was mainly associated with cerebrovascular risk (beta'-coefficient = 0.284, p < 0.0001) together with age, systolic blood pressure, total cholesterol, hs-CRP, body mass index, dietary poly unsaturated fatty acid/saturated fatty acid (PUFA/SFA), and HbA1C (r2 = 0.634, p = 0.044). The subjects were subdivided by their fasting glucose levels [normal fasting glucose: 70-99 mg/dL, n = 91 [NFG-control] and n = 27 [NFG-stroke]; higher fasting glucose: 100-125 mg/dL, n = 21 [HFG-control] and n = 14 [HFG-stroke]). In both controls and stroke patients, HFG groups show higher triglyceride, total- and LDL-cholesterol and lower HDL-cholesterol than NFG groups. Control-HFG group showed significantly higher levels of oxidative stress and inflammation than control-NFG group. Stroke-HFG group also showed significantly higher inflammatory levels than stroke-NFG group, moreover the highest among the groups. Additionally, stroke-NFG group consumed higher PUFA/SFA than stroke-HFG group. Fasting glucose may be a useful indicator for cerebrovascular risk in non-diabetic individuals which may be mediated by oxidative stress and inflammation status.
MeSH Terms
-
Blood Pressure
Body Mass Index
C-Reactive Protein
Cholesterol
Fasting*
Glucose*
Hemoglobin A, Glycosylated
Humans
Incidence
Inflammation*
Interleukin-6
Lipoproteins
Malondialdehyde
Oxidative Stress*
Stroke
Triglycerides
Tumor Necrosis Factor-alpha
C-Reactive Protein
Cholesterol
Glucose
Interleukin-6
Lipoproteins
Malondialdehyde
Triglycerides
Tumor Necrosis Factor-alpha
Figure
Cited by 1 articles
-
Glycated Hemoglobin and Cancer Risk in Korean Adults: Results from Korean Genome and Epidemiology Study
Ji Young Kim, Youn Sue Lee, Garam Jo, Min-Jeong Shin
Clin Nutr Res. 2018;7(3):170-177. doi: 10.7762/cnr.2018.7.3.170.
Reference
-
1. Santulli G. Epidemiology of cardiovascular disease in the 21st century: updated numbers and updated facts. J Cardiovasc Dis. 2013; 1:1–2.2. Centers for Disease Control and Prevention (US). National Center for Health Statistics. Leading causes of death [Internet]. 2013. cited 2013 June 20. Available from: http://www.cdc.gov/nchs/fastats/lcod.htm.3. World Health Organization. World Heart Federation. World Stroke Organization. Mendis S, Puska P, Norrving B, editors. Global atlas on cardiovascular disease prevention and control. Geneva: World Health Organization;2011.4. Statistics Korea. Cause of death statistics in 2013. Daejeon: Statistics Korea;2014.5. Tanne D, Koren-Morag N, Goldbourt U. Fasting plasma glucose and risk of incident ischemic stroke or transient ischemic attacks: a prospective cohort study. Stroke. 2004; 35:2351–2355.
Article6. Nielson C, Fleming RM. Blood glucose and cerebrovascular disease in nondiabetic patients. Angiology. 2007; 58:625–629.
Article7. Boden-Albala B, Cammack S, Chong J, Wang C, Wright C, Rundek T, Elkind MS, Paik MC, Sacco RL. Diabetes, fasting glucose levels, and risk of ischemic stroke and vascular events: findings from the Northern Manhattan Study (NOMAS). Diabetes Care. 2008; 31:1132–1137.
Article8. Rundek T, Gardener H, Xu Q, Goldberg RB, Wright CB, Boden-Albala B, Disla N, Paik MC, Elkind MS, Sacco RL. Insulin resistance and risk of ischemic stroke among nondiabetic individuals from the northern Manhattan study. Arch Neurol. 2010; 67:1195–1200.
Article9. Wright E Jr, Scism-Bacon JL, Glass LC. Oxidative stress in type 2 diabetes: the role of fasting and postprandial glycaemia. Int J Clin Pract. 2006; 60:308–314.
Article10. Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003; 52:2795–2804.
Article11. Schiekofer S, Andrassy M, Chen J, Rudofsky G, Schneider J, Wendt T, Stefan N, Humpert P, Fritsche A, Stumvoll M, Schleicher E, Häring HU, Nawroth PP, Bierhaus A. Acute hyperglycemia causes intracellular formation of CML and activation of ras, p42/44 MAPK, and nuclear factor kappaB in PBMCs. Diabetes. 2003; 52:621–633.
Article12. Jones SC, Saunders HJ, Qi W, Pollock CA. Intermittent high glucose enhances cell growth and collagen synthesis in cultured human tubulointerstitial cells. Diabetologia. 1999; 42:1113–1119.
Article13. Chae JS, Kim OY, Paik JK, Kang R, Seo WJ, Jeong TS, Sweeney G, Lee SH, Lee JH. Association of Lp-PLA(2) activity and LDL size with interleukin-6, an inflammatory cytokine and oxidized LDL, a marker of oxidative stress, in women with metabolic syndrome. Atherosclerosis. 2011; 218:499–506.
Article14. Ohnuki Y, Nagano R, Takizawa S, Takagi S, Miyata T. Advanced glycation end products in patients with cerebral infarction. Intern Med. 2009; 48:587–591.
Article15. Kim OY, Lim HH, Lee MJ, Kim JY, Lee JH. Association of fatty acid composition in serum phospholipids with metabolic syndrome and arterial stiffness. Nutr Metab Cardiovasc Dis. 2013; 23:366–374.
Article16. Song J, Kwon N, Lee MH, Ko YG, Lee JH, Kim OY. Association of serum phospholipid PUFAs with cardiometabolic risk: beneficial effect of DHA on the suppression of vascular proliferation/inflammation. Clin Biochem. 2014; 47:361–368.
Article17. Kwak JH, Paik JK, Kim HI, Kim OY, Shin DY, Kim HJ, Lee JH, Lee JH. Dietary treatment with rice containing resistant starch improves markers of endothelial function with reduction of postprandial blood glucose and oxidative stress in patients with prediabetes or newly diagnosed type 2 diabetes. Atherosclerosis. 2012; 224:457–464.
Article18. Shim JS, Oh KW, Suh I. A Study on Validity of a Semi-Quantitative Food Frequency Questionnaire for Korean Adults. Korean J Community Nutr. 2002; 7:484–494.19. Christian JL, Greger JL. Nutrition for living. Redwood City (CA): Benjamin/Cummings;1994.20. The American Dietetic Association. Handbook of clinical dietetics. 2nd ed. New Haven (CT): Yale University Press;1992.21. Sung J, Song YM, Ebrahim S, Lawlor DA. Fasting blood glucose and the risk of stroke and myocardial infarction. Circulation. 2009; 119:812–819.
Article22. Cao W, Ling Y, Wu F, Yang L, Cheng X, Dong Q. Higher fasting glucose next day after intravenous thrombolysis is independently associated with poor outcome in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2015; 24:100–103.
Article23. Vermeer SE, Sandee W, Algra A, Koudstaal PJ, Kappelle LJ, Dippel DW. Dutch TIA Trial Study Group. Impaired glucose tolerance increases stroke risk in nondiabetic patients with transient ischemic attack or minor ischemic stroke. Stroke. 2006; 37:1413–1417.
Article24. Larsson SC, Virtamo J, Wolk A. Dietary fats and dietary cholesterol and risk of stroke in women. Atherosclerosis. 2012; 221:282–286.
Article25. Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011; 58:2047–2067.26. Kim YJ, Kim OY, Cho Y, Chung JH, Jung YS, Hwang GS, Shin MJ. Plasma phospholipid fatty acid composition in ischemic stroke: importance of docosahexaenoic acid in the risk for intracranial atherosclerotic stenosis. Atherosclerosis. 2012; 225:418–424.
Article27. Yakoob MY, Shi P, Hu FB, Campos H, Rexrode KM, Orav EJ, Willett WC, Mozaffarian D. Circulating biomarkers of dairy fat and risk of incident stroke in U.S. men and women in 2 large prospective cohorts. Am J Clin Nutr. 2014; 100:1437–1447.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intermittent Fasting in Diabetic Patients
- Gamma-tocopherol ameliorates hyperglycemia-induced hepatic inflammation associated with NLRP3 inflammasome in alloxan-induced diabetic mice
- Lipid Accumulation Product Is Associated with Insulin Resistance, Lipid Peroxidation, and Systemic Inflammation in Type 2 Diabetic Patients
- Effects of Short Term Antioxidant Cocktail Supplementation on the Oxidative Stress and Inflammatory Response of Renal Inflammation in Diabetic Mice
- Oxidative Stress in Pancreatic Islet beta-cells Exposed to High Glucose Concentration