Korean J Nutr.
2009 Dec;42(8):673-681.
Effects of Short Term Antioxidant Cocktail Supplementation on the Oxidative Stress and Inflammatory Response of Renal Inflammation in Diabetic Mice
- Affiliations
-
- 1Department of Food and Nutrition, Research Institute of Human Ecology, Kyunghee University, Seoul 130-701, Korea. ylim@khu.ac.kr
Abstract
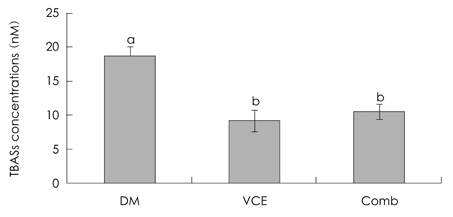
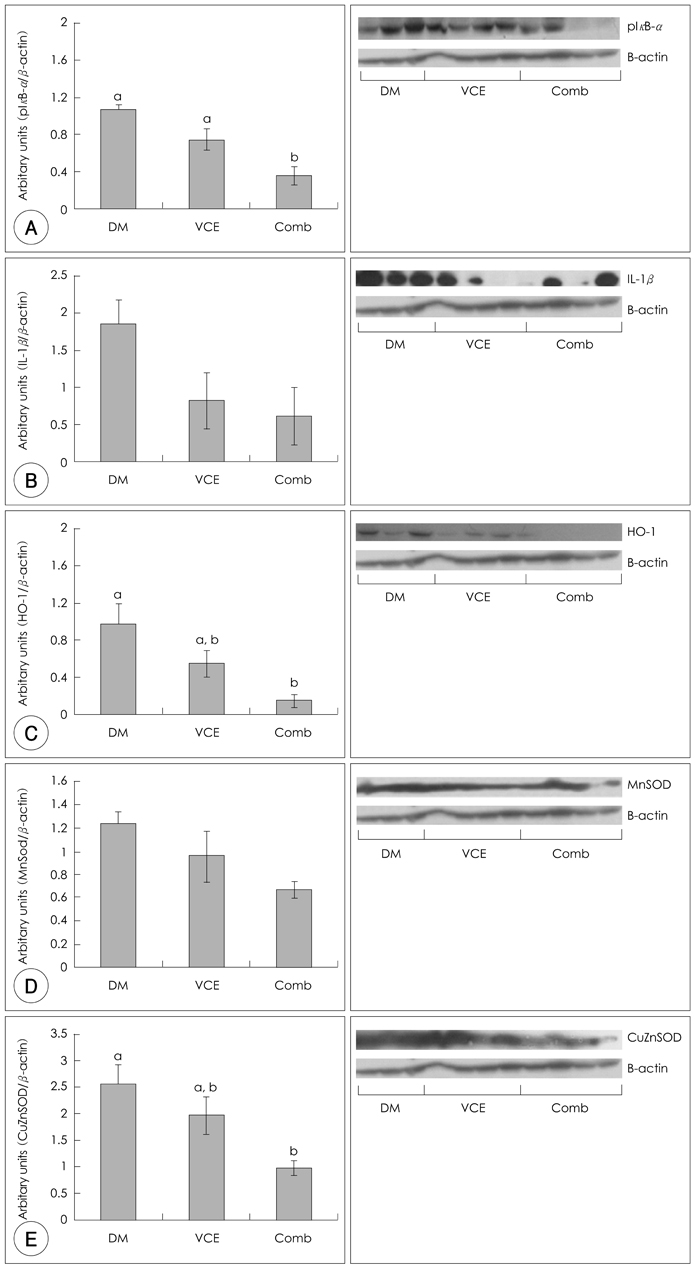
- Diabetes mellitus is a multifactorial disease. Particularly, diabetic nephropathy is a serious complication for diabetic patients, yet the precise mechanisms that underline the initial stage of diabetic renal inflammation remain unknown. However, oxidative stress induced by hyperglycemia in diabetes is implicated in diabetic renal disease. We hypothesized that dietary supplementation of antioxidants either VCE (0.5% VC + 0.5% VE) or Comb (0.5% VC + 0.5% VE + 2.5% N-acetylcysteine) improves acute diabetic renal inflammation through modulation of blood glucose levels and antioxidant and anti-inflammatory responses. Experimental animals (5.5 weeks old female ICR) used were treated with alloxan (180 mg/kg) once. When fasting blood glucose levels were higher than 250 mg/dL, mice were divided into 3 groups fed different levels of antioxidant supplementation, DM (diabetic mice fed AIN 93G purified rodent diet); VCE (diabetic mice fed 0.5% vitamin C and 0.5% vitamin E supplemented diet); Comb (diabetic mice fed 0.5% vitamin C, 0.5% vitamin E and 2.5% N-acetylcysteine supplemented diet), for 10 days and then sacrificed. Body weights were measured once a week and blood glucose levels were monitored twice a week. Lipid peroxidation products, thiobarbituric acid reacting substances were measured in kidney. NF-kappaB activation was indirectly demonstrated by pIkappaB-alpha and expressions of selective inflammatory and oxidative stress markers including antioxidant enzymes were also determined. Dietary antioxidant supplementation improved levels of blood glucose as well as kidney lipid peroxi-dation. Dietary antioxidant supplementation improved NF-kappaB activation and protein expression of HO-1, but not mRNA expression levels in diabetic mice fed Comb diet. In contrast, the mRNA and protein expression of CuZnSOD was decreased in diabetic mice fed Comb diet. However, antioxidant supplementation did not improve mRNA and protein expressions of IL-1beta and MnSOD in diabetic mice. These findings demonstrate that acute diabetic renal inflammation was associated with altered inflammatory and antioxidant responses and suggest that antioxidant cocktail supplementation may have beneficial effects on early stage of diabetic nephropathy through modulation of blood glucose levels and antioxidant enzyme expressions.
MeSH Terms
-
Acetylcysteine
Alloxan
Antioxidants
Ascorbic Acid
Blood Glucose
Body Weight
Diabetes Mellitus
Diabetic Nephropathies
Diet
Dietary Supplements
Fasting
Female
Humans
Hyperglycemia
Inflammation
Kidney
Lipid Peroxidation
Mice
NF-kappa B
Oxidative Stress
RNA, Messenger
Rodentia
Thiobarbiturates
Vitamin E
Vitamins
Acetylcysteine
Alloxan
Antioxidants
Ascorbic Acid
Blood Glucose
NF-kappa B
RNA, Messenger
Thiobarbiturates
Vitamin E
Vitamins
Figure
Reference
-
1. Brem H, Tomic-Canic M, Entero H, Hanflik AM, Wang VM, Fallon JT, Ehrlich HP. The synergism of age and db/db genotype impairs wound healing. Exp Gerontol. 2007. 42(6):523–531.
Article2. Choe GH, No HJ, Kim BS, Kang SU, Han DS, Lee HY. Inducible Nitric Oxide Synthase (iNOS) is Increased in Diabetic Rat Glomeruli: Role of Angiotensin 2 (A2). Korean J Nephol. 2007. 22(4):366–373.3. Ha HJ, Lee HB. Reactive oxygen species and diabetic nephropathy. Korean J Nephol. 2004. 23(2):425–427.4. Kim HY, Jang JH, Han SM, Ahn KS, Park SB, Kim HCl, Park KK. Expression of Transforming Growth Factor-beta1 Spontaneously Developed Diabetic Rats. Korean J Nephol. 2003. 22(2):165–173.5. Kim IJ, Kim BW, Ha SW, Kim DW, Kim YK, Kim TH, Park JY, Yu HJ, Lee MK, Lee IK, Cha BY. Hyperglycemia and oxidative stress. Biology Research Information Center. 2003. 5(6):1–7.6. Lee H, Yu MR, Yang Y, Jiang Z, Ha H. Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J Am Soc Nephrol. 2003. 14(8):241–245.
Article7. Bohlender JM, Franke S, Stein G, Wolf G. Advanced glycation end products and the kidney. Am J Physiol Renal Physiol. 2005. 289:F645–F659.
Article8. Navarro JF, Mora C. Role of inflammation in diabetic complications. Nephrol Dial Transplant. 2005. 20(12):2601–2604.
Article9. Han DC. Role of VEGF in diabetic nephropathy. Korean J Nephrol. 2000. 19(2):118–122.10. Ho E, Chen G, Bray TM. Supplementation of N-acetylcysteine inhibits NFkappaB activation and protects against alloxan-induced diabetes in CD-1 mice. Faseb J. 1999. 13(13):1845–1854.
Article11. Musalmah M, Fairuz AH, Gapor MT, Ngah WZ. Effect of vitamin E on plasma malondialdehyde, antioxidant enzyme levels and the rates of wound closures during wound healing in normal and diabetic rats. Asia Pac J Clin Nutr. 2002. 11(7):S448–S451.
Article12. Lee E, Lee , MY , Hong SW, Chung CH, Hong SY. Blockade of oxidative stress by vitamin C ameliorates albuminuria and renal sclerosis in experimental diabetic rats. Yonsei Med J. 2007. 48(5):847–855.
Article13. Ueno Y, Kizaki M, Nakagiri R, Kamiya T, Sumi H, Osawa T. Dietary glutathione protects rats from diabetic nephropathy and neuropathy. J Nutr. 2002. 132(5):897–900.
Article14. Ho E, Quan N, Tsai YH, Lai W, Bray TM. Dietary zinc supplementation inhibits NFkappaB activation and protects against chemically induced diabetes in CD1 mice. Exp Biol Med (Maywood). 2001. 226(2):103–111.
Article15. Kaneto H, Kajimoto Y, Miyagawa J, Matsuoka T, Fujitani Y, Umayahara Y, Hanafusa T, Matsuzawa Y, Yamasaki Y, Hori M. Benefical Effects of Antioxidants in Diabetes: Possible protection of pancreatic β-cell against glucose toxicity. Diabetes. 1999. 48:2398–2406.
Article16. Kedziora-Kornatowska K, Szram S, Kornatowski T, Szadujkis-Szadurski L, Kedziora J, Bartosz G. Effect of vitamin E and vitamin C supplementation on antioxidative state and renal glomerular basement membrane thickness in diabetic kidney. Nephron Exp Nephrol. 2003. 95(4):e134–e143.
Article17. Inada A, Kanamori H, Arai H, Akashi T, Araki M, Weir GC, Fukatsu A. A model for diabetic nephropathy: advantages of the inducible cAMP early repressor transgenic mouse over the streptozotocin-induced diabetic mouse. J Cell Physiol. 2008. 215(2):383–391.
Article18. Yue KKM, Chung WS, Leung AWN, Cheng CHK. Redox changes precede the occurrence of oxidative stress in eyes and arota, but not in kidneys of diabetic rats. Life Sci. 2003. 73(20):2557–2570.
Article19. Garg MC, Ojha S, Bansal DD. Antioxidant status of streptozotocin diabetic rats. Indian J Exp Biol. 1996. 34(3):264–266.20. Santini SA, Marra G, Giardina B, Cotroneo P, Mordente A, Martorana GE, Manto A, Ghirlanda G. Defective plasma antioxidant defenses and enhanced susceptibility to lipid peroxidation in uncomplicated IDDM. Diabetes. 1997. 46(11):1853–1858.
Article21. Takasu N, Komiya I, Asawa T, Nagasawa Y, Yamada T. Streptozocin- and alloxan-induced H2O2 generation and DNA fragmentation in pancreatic islets: H2O2 as mediator for DNA fragmentation. Diabetes. 1991. 40(9):1141–1145.
Article22. Odetti P, Pesce C, Traverso N, Menini S, Maineri EP, Cosso L, Valentini S, Patriarca S, Cottalasso D, Marinari UM, Pronzato MA. Comparative trial of N-acetyl-cysteine, taurine, and oxerutin on skin and kidney damage in long-term experimental diabetes. Diabetes. 2003. 52(2):499–505.
Article23. Kuhad A, Chopra K. Attenuation of diabetic nephropathy by tocotrienol: involvement of NFkB signaling pathway. Life Sci. 2009. 84(9-10):296–301.
Article24. Hayashi K, Haneda M, Koya D, Maeda S, Isshiki K, Kikkawa R. Enhancement of glomerular heme oxygenase-1 expression in diabetic rats. Diabetes Res Clin Pract. 2001. 52(2):85–96.
Article25. Vogt BA, Shanley TP, Croatt A, Alam J, Johnson KJ, Nath KA. Glomerular inflammation induces resistance to tubular injury in the rat. A novel form of acquired, heme oxygenase-dependent resistance to renal injury. J Clin Invest. 1996. 98(9):2139–2145.
Article26. Koya D, Hayashi K, Kitada M, Kashiwagi A, Kikkawa R, Haneda M. Effects of antioxidants in diabetes-induced oxidative stress in the glomeruli of diabetic rats. J Am Soc Nephrol. 2003. 14:S250–S253.
Article27. Emerit J, Michelson AM. [Free radicals in medicine and biology]. Sem Hop. 1982. 58(45):2670–2675.28. Kim MJ, Cho SY, Lee MK, Shin KH. Effects of Aralia elata Water Extracts on Activities of Hepatic Oxygen Free Radical Generating and Scavenging Enzymes in Streptozotocin-Induced Diabetic Rats. J Korean Soc Food Sci Nutr. 2004. 33(4):653–658.
Article29. Wolff SP, Jiang ZY, Hunt JV. Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic Biol Med. 1991. 10(5):339–352.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Similarities and differences between alpha-tocopherol and gamma-tocopherol in amelioration of inflammation, oxidative stress and pre-fibrosis in hyperglycemia induced acute kidney inflammation
- Gamma-tocopherol ameliorates hyperglycemia-induced hepatic inflammation associated with NLRP3 inflammasome in alloxan-induced diabetic mice
- Effects of S-allylcysteine on Oxidative Stress in Streptozotocin-Induced Diabetic Rats
- Green perilla leaf extract ameliorates long-term oxidative stress induced by a high-fat diet in aging mice
- Pan-Nox inhibitor treatment improves renal function in aging murine diabetic kidneys