Nutr Res Pract.
2022 Oct;16(5):549-564. 10.4162/nrp.2022.16.5.549.
Green perilla leaf extract ameliorates long-term oxidative stress induced by a high-fat diet in aging mice
- Affiliations
-
- 1Department of Food Science and Human Nutrition, Jeonbuk National University, Jeonju 54896, Korea
- 2Department of Nutrition, University of Massachusetts, Amherst, MA 01007, USA
- 3Food and Policy Division, Wanju County Office, Wanju 55352, Korea
- 4K-Food Research Center, Jeonbuk National University, Jeonju 54896, Korea
- KMID: 2534115
- DOI: http://doi.org/10.4162/nrp.2022.16.5.549
Abstract
- BACKGROUND/OBJECTIVES
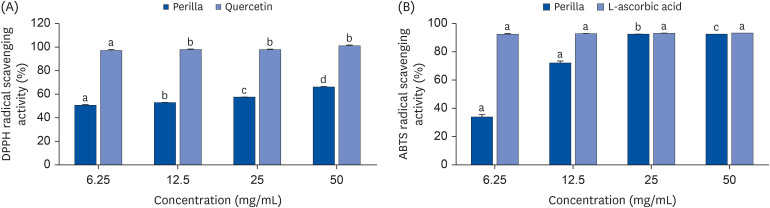
Oxidative stress is caused by an imbalance between harmful free radicals and antioxidants. Long-term oxidative stress can lead to an “exhausted” status of antioxidant defense system triggering development of metabolic syndrome and chronic inflammation. Green perilla (Perilla frutescens) is commonly used in Asian cuisines and traditional medicine in southeast Asia. Green perilla possesses numerous beneficial effects including anti-inflammatory and antioxidant functions. To investigate the potentials of green perilla leaf extract (PE) on oxidative stress, we induced oxidative stress by high-fat diet (HFD) in aging mice.
MATERIALS/METHODS
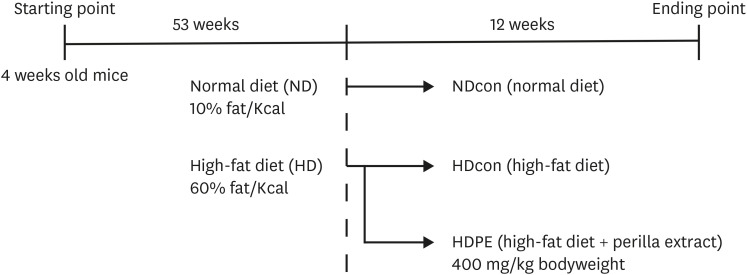
C57BL/6J male mice were fed HFD continuously for 53 weeks. Then, mice were divided into three groups for 12 weeks: a normal diet fed reference group (NDcon), high-fat diet fed group (HDcon), and high-fat diet PE treated group (HDPE, 400 mg/kg of body weight). Biochemical analyses of serum and liver tissues were performed to assess metabolic and inflammatory damage and oxidative status. Hepatic gene expression of oxidative stress and inflammation related enzymes were evaluated by quantitative real-time polymerase chain reaction (qRT-PCR).
RESULTS
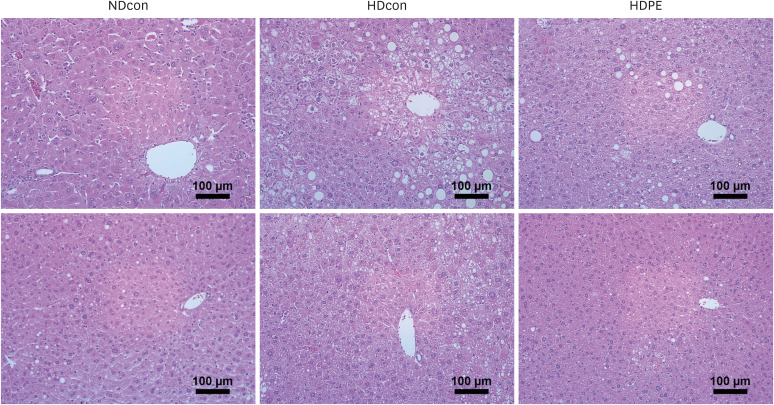
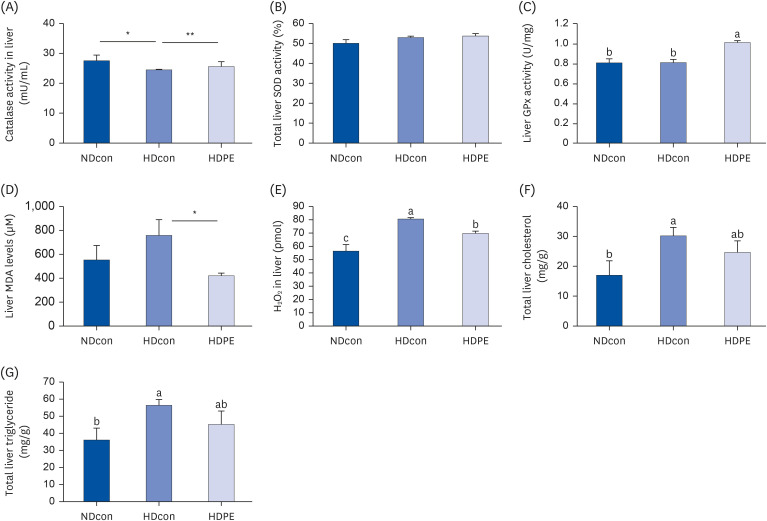
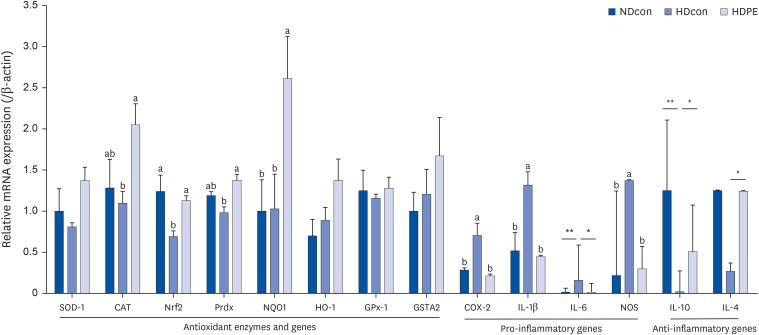
PE improved hepatopathology. PE also improved the lipid profiles and antioxidant enzymes, including hepatic glutathione peroxidase (GPx) and superoxide dismutase (SOD) and catalase (CAT) in serum and liver. Hepatic gene expressions of antioxidant and antiinflammatory related enzymes, such as SOD-1, CAT, interleukin 4 (IL-4) and nuclear factor erythroid 2-related factor (Nrf2) were significantly enhanced by PE. PE also reduced the levels of hydrogen peroxide (H 2 O 2 ) and malondialdehyde (MDA) in the serum and liver; moreover, PE suppressed hepatic gene expression involved in pro-inflammatory response; Cyclooxygenase-2 (COX-2), nitric oxide synthase (NOS), interleukin 1 beta (IL-1β), and interleukin 6 (IL-6).
CONCLUSIONS
This research opens opportunities for further investigations of PE as a functional food and possible anti-aging agent due to its attenuative effects against oxidative stress, resulting from HFD and aging in the future.
Keyword
Figure
Reference
-
1. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013; 153:1194–1217. PMID: 23746838.
Article2. Said MA, Aiman IA. Oxidative stress versus antioxidants. Am J Biosci Bioeng. 2014; 2:60–71.3. Camps J, García-Heredia A. Introduction: oxidation and inflammation, a molecular link between non-communicable diseases. Adv Exp Med Biol. 2014; 824:1–4. PMID: 25038988.
Article4. Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018; 13:757–772. PMID: 29731617.
Article5. Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev. 2017; 2017:8416763. PMID: 28819546.
Article6. Kelly DS, Betim CC, Talita CC, Giovana AG, Laura MM, Rizzato PJ. Antioxidant activity of aqueous extract of passion fruit (Passiflora edulis) leaves: in vitro and in vivo study. Food Res Int. 2013; 53:882–890.7. Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996; 273:59–63. PMID: 8658196.
Article8. Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot. 2003; 91:179–194. PMID: 12509339.
Article9. Kartha GK, Moshal KS, Sen U, Joshua IG, Tyagi N, Steed MM, Tyagi SC. Renal mitochondrial damage and protein modification in type-2 diabetes. Acta Diabetol. 2008; 45:75–81. PMID: 18292963.
Article10. Bruce KD, Cagampang FR, Argenton M, Zhang J, Ethirajan PL, Burdge GC, Bateman AC, Clough GF, Poston L, Hanson MA, et al. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology. 2009; 50:1796–1808. PMID: 19816994.
Article11. Yokota T, Kinugawa S, Hirabayashi K, Matsushima S, Inoue N, Ohta Y, Hamaguchi S, Sobirin MA, Ono T, Suga T, et al. Oxidative stress in skeletal muscle impairs mitochondrial respiration and limits exercise capacity in type 2 diabetic mice. Am J Physiol Heart Circ Physiol. 2009; 297:H1069–H1077. PMID: 19617406.
Article12. Ballal K, Wilson CR, Harmancey R, Taegtmeyer H. Obesogenic high fat western diet induces oxidative stress and apoptosis in rat heart. Mol Cell Biochem. 2010; 344:221–230. PMID: 20676734.
Article13. Moreno-Fernández S, Garcés-Rimón M, Vera G, Astier J, Landrier JF, Miguel M. High fat/high glucose diet induces metabolic syndrome in an experimental rat model. Nutrients. 2018; 10:1502.
Article14. Ceriello A. Oxidative stress and diabetes-associated complications. Endocr Pract. 2006; 12(Suppl 1):60–62. PMID: 16627383.
Article15. Giugliano D, Ceriello A, Paolisso G. Diabetes mellitus, hypertension, and cardiovascular disease: which role for oxidative stress? Metabolism. 1995; 44:363–368. PMID: 7885282.
Article16. Jun HI, Kim BT, Song GS, Kim YS. Structural characterization of phenolic antioxidants from purple perilla (Perilla frutescens var. acuta) leaves. Food Chem. 2014; 148:367–372. PMID: 24262570.
Article17. Hong E, Park KH, Kim GH. Phenolic-enriched fractions from Perilla frutescens var. acuta: determinating rosmarinic acid and antioxidant activity. J Food Biochem. 2011; 35:1637–1645.
Article18. Tian SL. Perilla frutescens detoxifies the toxicity of fish and crab. Zhong Hua Yang Sheng Bao Jian. 2012; 9:71.19. The State Pharmacopoeia Commission of P. R. China. Pharmacopoeia of the People’s Republic of China (PPRC). Beijing: Chinese Medical Science Press;2015. p. 338–340.20. Huang BP, Lin CH, Chen YC, Kao SH. Anti-inflammatory effects of Perilla frutescens leaf extract on lipopolysaccharide-stimulated RAW264.7 cells. Mol Med Rep. 2014; 10:1077–1083. PMID: 24898576.
Article21. Heo JC, Nam DY, Seo MS, Lee SH. Alleviation of atopic dermatitis-related symptoms by Perilla frutescens Britton. Int J Mol Med. 2011; 28:733–737. PMID: 21811759.
Article22. Zhu F, Asada T, Sato A, Koi Y, Nishiwaki H, Tamura H. Rosmarinic acid extract for antioxidant, antiallergic, and α-glucosidase inhibitory activities, isolated by supramolecular technique and solvent extraction from Perilla leaves. J Agric Food Chem. 2014; 62:885–892. PMID: 24400891.
Article23. Peng Y, Ye J, Kong J. Determination of phenolic compounds in Perilla frutescens L. by capillary electrophoresis with electrochemical detection. J Agric Food Chem. 2005; 53:8141–8147. PMID: 16218656.
Article24. Osakabe N, Yasuda A, Natsume M, Sanbongi C, Kato Y, Osawa T, Yoshikawa T. Rosmarinic acid, a major polyphenolic component of Perilla frutescens, reduces lipopolysaccharide (LPS)-induced liver injury in D-galactosamine (D-GalN)-sensitized mice. Free Radic Biol Med. 2002; 33:798–806. PMID: 12208367.
Article25. Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S, Ju YH. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica . J Food Drug Anal. 2014; 22:296–302. PMID: 28911418.
Article26. Bektas T, Dimitra D, Atalay S, Munevver S, Moschos P. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem. 2005; 90:333–340.27. Zheleva-Dimitrova D, Nedialkov P, Kitanov G. Radical scavenging and antioxidant activities of methanolic extracts from Hypericum species growing in Bulgaria. Pharmacogn Mag. 2010; 6:74–78. PMID: 20668569.
Article28. Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014; 4:177. PMID: 24454289.
Article29. Pichiah PB, Cha YS. Salicornia herbacea prevents weight gain and hepatic lipid accumulation in obese ICR mice fed a high-fat diet. J Sci Food Agric. 2015; 95:3150–3159. PMID: 25523516.
Article30. Hong E, Kim GH. Comparison of extraction conditions for phenolic, flavonoid content and determination of rosmarinic acid from Perilla frutescens var. acuta. Int J Food Sci Technol. 2010; 45:1353–1359.
Article31. Lee YH, Kim B, Kim S, Kim MS, Kim H, Hwang SR, Kim K, Lee JH. Characterization of metabolite profiles from the leaves of green perilla (Perilla frutescens) by ultra high performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry and screening for their antioxidant properties. J Food Drug Anal. 2017; 25:776–788. PMID: 28987353.
Article32. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016; 7:27–31. PMID: 27057123.
Article33. Ebrahimzadeh Attari V, Ostadrahimi A, Asghari Jafarabadi M, Mehralizadeh S, Mahluji S. Changes of serum adipocytokines and body weight following Zingiber officinale supplementation in obese women: a RCT. Eur J Nutr. 2016; 55:2129–2136. PMID: 26318445.
Article34. Webster GF, Webster TG, Grimes LR. Laboratory tests in patients treated with isotretinoin: occurrence of liver and muscle abnormalities and failure of AST and ALT to predict liver abnormality. Dermatol Online J. 2017; 23:13030/qt7rv7j80p.
Article35. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004; 114:1752–1761. PMID: 15599400.
Article36. Raval J, Lyman S, Nitta T, Mohuczy D, Lemasters JJ, Kim JS, Behrns KE. Basal reactive oxygen species determine the susceptibility to apoptosis in cirrhotic hepatocytes. Free Radic Biol Med. 2006; 41:1645–1654. PMID: 17145552.
Article37. Cichoż-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. 2014; 20:8082–8091. PMID: 25009380.
Article38. Kim MJ, Kim HK. Perilla leaf extract ameliorates obesity and dyslipidemia induced by high-fat diet. Phytother Res. 2009; 23:1685–1690. PMID: 19444921.
Article39. Bruunsgaard H, Ladelund S, Pedersen AN, Schroll M, Jørgensen T, Pedersen BK. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin Exp Immunol. 2003; 132:24–31. PMID: 12653832.
Article40. Thomas SS, Cha YS, Kim KA. Effect of vegetable oils with different fatty acid composition on high-fat diet-induced obesity and colon inflammation. Nutr Res Pract. 2020; 14:425–437. PMID: 33029284.
Article41. Thomas SS, Cha YS, Kim KA. Perilla oil alleviates high-fat diet-induced inflammation in the colon of mice by suppressing nuclear factor-kappa B activation. J Med Food. 2020; 23:818–826. PMID: 32552354.
Article42. Chung HY, Lee EK, Choi YJ, Kim JM, Kim DH, Zou Y, Kim CH, Lee J, Kim HS, Kim ND, et al. Molecular inflammation as an underlying mechanism of the aging process and age-related diseases. J Dent Res. 2011; 90:830–840. PMID: 21447699.
Article43. Kim J, Vaish V, Feng M, Field K, Chatzistamou I, Shim M. Transgenic expression of cyclooxygenase-2 (COX2) causes premature aging phenotypes in mice. Aging (Albany NY). 2016; 8:2392–2406. PMID: 27750221.
Article44. Pandareesh MD, Chauhan V, Chauhan A. Walnut supplementation in the diet reduces oxidative damage and improves antioxidant status in transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis. 2018; 64:1295–1305. PMID: 30040727.
Article45. Mehta SK, Gowder SJ. Members of Antioxidant Machinery and Their Functions. Basic Principles and Clinical Significance of Oxidative Stress. London: IntechOpen Ltd.;2015. p. 59–85.46. Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013; 53:401–426. PMID: 23294312.
Article47. Espinosa-Diez C, Miguel V, Mennerich D, Kietzmann T, Sánchez-Pérez P, Cadenas S, Lamas S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015; 6:183–197. PMID: 26233704.
Article48. Bryan HK, Olayanju A, Goldring CE, Park BK. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem Pharmacol. 2013; 85:705–717. PMID: 23219527.
Article49. Kharaeva Z, Gostova E, De Luca C, Raskovic D, Korkina L. Clinical and biochemical effects of coenzyme Q(10), vitamin E, and selenium supplementation to psoriasis patients. Nutrition. 2009; 25:295–302. PMID: 19041224.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of combined mulberry leaf and fruit extract on liver and skin cholesterol transporters in high fat diet-induced obese mice
- Anti-obesity effects of Rapha diet(R) preparation in mice fed a high-fat diet
- Inhibitory effects of Doenjang, Korean traditional fermented soybean paste, on oxidative stress and inflammation in adipose tissue of mice fed a high-fat diet
- The Neuro-Protective Effect of the Methanolic Extract of Perilla frutescens var. japonica and Rosmarinic Acid against H2O2-Induced Oxidative Stress in C6 Glial Cells
- The Effect of Grape Seed Oil, Perilla Oil, or Corn Oil-Containing Diet on Lipid Patterns in Rats and Fatty-Acid Composition in Their Liver Tissues