Yonsei Med J.
2013 Jul;54(4):845-853. 10.3349/ymj.2013.54.4.845.
The Effects of Prucalopride on Postoperative Ileus in Guinea Pigs
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. hjpark21@yuhs.ac
- KMID: 2158217
- DOI: http://doi.org/10.3349/ymj.2013.54.4.845
Abstract
- PURPOSE
Postoperative ileus (POI) is an impairment of coordinated gastrointestinal (GI) motility that develops as a consequence of abdominal surgery and is a major factor contributing to patient morbidity and prolonged hospitalization. The aim of this study was to investigate the effects of different 5-hydroxytryptamine 4 (5-HT4) receptor agonists, which stimulate excitatory pathways, on a POI model.
MATERIALS AND METHODS
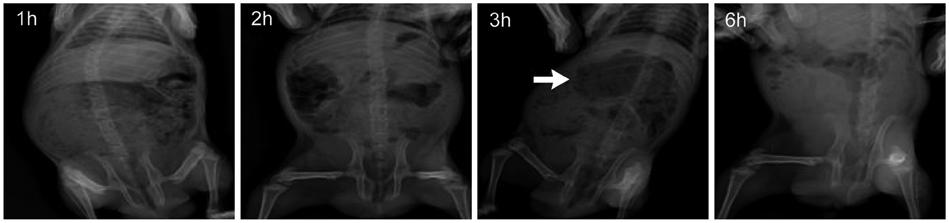
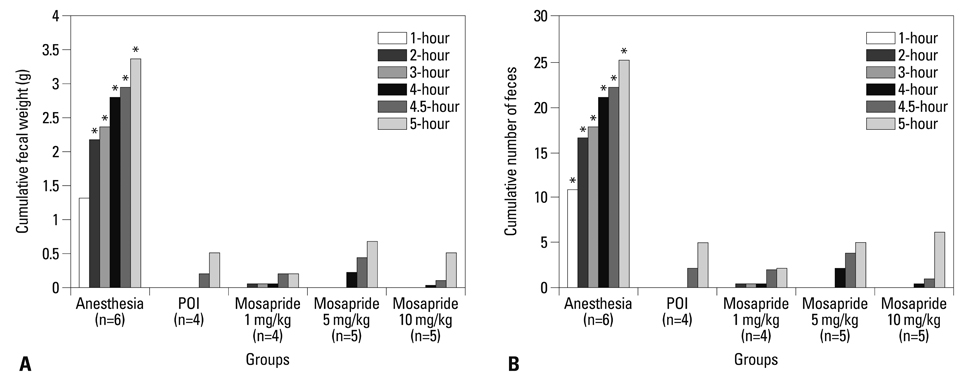
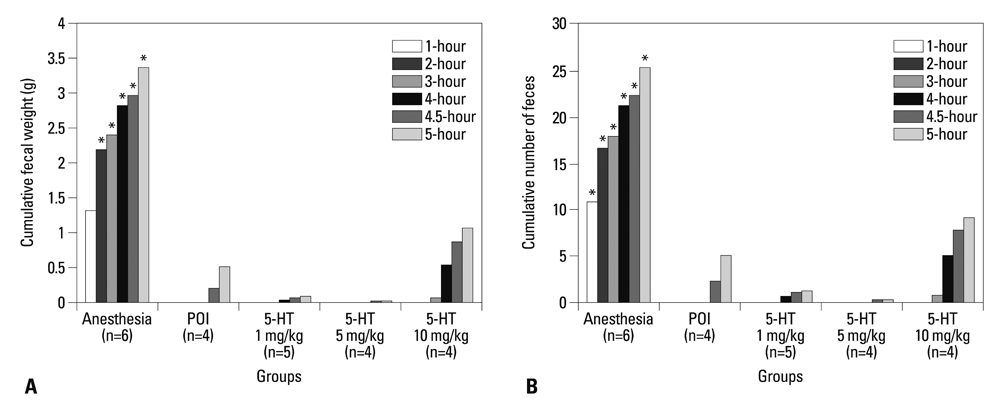
The experimental model of POI in guinea pigs was created by laparotomy, gentle manipulation of the cecum for 60 seconds, and closure by suture, all under anesthesia. Different degrees of restoration of GI transit were measured by the migration of charcoal. Colonic transit was indirectly assessed via measurement of fecal pellet output every hour for 5 hours after administration of various doses of mosapride, tegaserod, prucalopride, and 5-HT.
RESULTS
Charcoal transit assay showed that various 5-HT4 receptor agonists can accelerate delayed upper GI transit in a dose-dependent manner. However, fecal pellet output assay suggested that only prucalopride had a significant effect in accelerating colonic motility in POI.
CONCLUSION
Although mosapride, tegaserod, and prucalopride produce beneficial effects to hasten upper GI transit in the POI model, prucalopride administered orally restores lower GI transit as well as upper GI transit after operation in a conscious guinea pig. This drug may serve as a useful candidate for examination in a clinical trial for POI.
MeSH Terms
-
Administration, Oral
Animals
Benzamides/pharmacology
Benzofurans/administration & dosage/*pharmacology
Charcoal/pharmacokinetics
Colon/drug effects
Dose-Response Relationship, Drug
Gastrointestinal Motility/*drug effects
Guinea Pigs
Ileus/*surgery
Indoles/pharmacology
Laparotomy
Male
Morpholines/pharmacology
Postoperative Complications/drug therapy
Serotonin/pharmacology
Serotonin 5-HT4 Receptor Agonists/*pharmacology
Benzamides
Benzofurans
Indoles
Morpholines
Serotonin 5-HT4 Receptor Agonists
Charcoal
Serotonin
Figure
Reference
-
1. Delaney CP, Senagore AJ, Viscusi ER, Wolff BG, Fort J, Du W, et al. Postoperative upper and lower gastrointestinal recovery and gastrointestinal morbidity in patients undergoing bowel resection: pooled analysis of placebo data from 3 randomized controlled trials. Am J Surg. 2006; 191:315–319.
Article2. Greenwood-Van Meerveld B. Emerging drugs for postoperative ileus. Expert Opin Emerg Drugs. 2007; 12:619–626.
Article3. Bauer AJ, Boeckxstaens GE. Mechanisms of postoperative ileus. Neurogastroenterol Motil. 2004; 16:Suppl 2. 54–60.
Article4. Boeckxstaens GE, de Jonge WJ. Neuroimmune mechanisms in postoperative ileus. Gut. 2009; 58:1300–1311.
Article5. De Winter BY, Boeckxstaens GE, De Man JG, Moreels TG, Schuurkes JA, Peeters TL, et al. Effect of different prokinetic agents and a novel enterokinetic agent on postoperative ileus in rats. Gut. 1999; 45:713–718.
Article6. Briejer MR, Bosmans JP, Van Daele P, Jurzak M, Heylen L, Leysen JE, et al. The in vitro pharmacological profile of prucalopride, a novel enterokinetic compound. Eur J Pharmacol. 2001; 423:71–83.
Article7. Schuurkes J, Meulemans A, Briejer M, De Ridder W, De Winter P. The enterokinetic R093877 normalizes opioid-delayed fecal pellet propulsion in the isolated guinea-pig distal colon. Gastroenterology. 1998; 114:A835.
Article8. Briejer MR, Prins NH, Schuurkes JA. Effects of the enterokinetic prucalopride (R093877) on colonic motility in fasted dogs. Neurogastroenterol Motil. 2001; 13:465–472.
Article9. Qi HB, Luo JY, Liu X. Effect of enterokinetic prucalopride on intestinal motility in fast rats. World J Gastroenterol. 2003; 9:2065–2067.
Article10. Camilleri M, Kerstens R, Rykx A, Vandeplassche L. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med. 2008; 358:2344–2354.
Article11. Tack J, van Outryve M, Beyens G, Kerstens R, Vandeplassche L. Prucalopride (Resolor) in the treatment of severe chronic constipation in patients dissatisfied with laxatives. Gut. 2009; 58:357–365.
Article12. Camilleri M, Beyens G, Kerstens R, Robinson P, Vandeplassche L. Safety assessment of prucalopride in elderly patients with constipation: a double-blind, placebo-controlled study. Neurogastroenterol Motil. 2009; 21:1256-e117.
Article13. Camilleri M, Van Outryve MJ, Beyens G, Kerstens R, Robinson P, Vandeplassche L. Clinical trial: the efficacy of open-label prucalopride treatment in patients with chronic constipation-follow-up of patients from the pivotal studies. Aliment Pharmacol Ther. 2010; 32:1113–1123.
Article14. Sloots CE, Rykx A, Cools M, Kerstens R, De Pauw M. Efficacy and safety of prucalopride in patients with chronic noncancer pain suffering from opioid-induced constipation. Dig Dis Sci. 2010; 55:2912–2921.
Article15. Emmanuel AV, Kamm MA, Roy AJ, Kerstens R, Vandeplassche L. Randomised clinical trial: the efficacy of prucalopride in patients with chronic intestinal pseudo-obstruction--a double-blind, placebo-controlled, cross-over, multiple n = 1 study. Aliment Pharmacol Ther. 2012; 35:48–55.
Article16. Mendzelevski B, Ausma J, Chanter DO, Robinson P, Kerstens R, Vandeplassche L, et al. Assessment of the cardiac safety of prucalopride in healthy volunteers: a randomized, double-blind, placebo-and positive-controlled thorough QT study. Br J Clin Pharmacol. 2012; 73:203–209.
Article17. De Maeyer JH, Lefebvre RA, Schuurkes JA. 5-HT4 receptor agonists: similar but not the same. Neurogastroenterol Motil. 2008; 20:99–112.
Article18. Manabe N, Wong BS, Camilleri M. New-generation 5-HT4 receptor agonists: potential for treatment of gastrointestinal motility disorders. Expert Opin Investig Drugs. 2010; 19:765–775.
Article19. Mine Y, Yoshikawa T, Oku S, Nagai R, Yoshida N, Hosoki K. Comparison of effect of mosapride citrate and existing 5-HT4 receptor agonists on gastrointestinal motility in vivo and in vitro. J Pharmacol Exp Ther. 1997; 283:1000–1008.20. Nguyen A, Camilleri M, Kost LJ, Metzger A, Sarr MG, Hanson RB, et al. SDZ HTF 919 stimulates canine colonic motility and transit in vivo. J Pharmacol Exp Ther. 1997; 280:1270–1276.21. Yeh YC, Klinger EV, Reddy P. Pharmacologic options to prevent postoperative ileus. Ann Pharmacother. 2009; 43:1474–1485.
Article22. Kim HS, Choi EJ, Park H. The effect of mosapride citrate on proximal and distal colonic motor function in the guinea-pig in vitro. Neurogastroenterol Motil. 2008; 20:169–176.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of anesthesia on the electrically-evoked middle latency responses on guinea pigs
- Experimental Dermatitis by Pityrosporum ovale in Guinea Pigs
- Electron microseopic study on the histopathological changes of the periodontal tissue of pressure side following experimmental tooth movement in guinea pigs
- In vivo Expansion of Contact Sensitivity Transferred by Allogeneic Spleen Cells in Guinea Pigs
- The Comparison of Cellular Immune Reactions in Mycobacterium Tuberculosis-Inoculated Guinea Pigs and Rabbits