J Korean Med Sci.
2013 Jan;28(1):80-86. 10.3346/jkms.2013.28.1.80.
Early Response to Bortezomib Combined Chemotherapy Can Help Predict Survival in Patients with Multiple Myeloma Who Are Ineligible for Stem Cell Transplantation
- Affiliations
-
- 1Department of Internal Medicine, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea.
- 2Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Division of Hematology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Hallym University Sacred Heart Hospital, Anyang, Korea.
- 5Department of Internal Medicine, St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea.
- 6Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 7Hematology-Oncology Clinic, National Cancer Center, Goyang, Korea.
- 8Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea.
- 9Department of Internal Medicine, Gachon University Gil Hospital, Incheon, Korea.
- 10Department of Hemato-Oncology, Yeungnam University College of Medicine, Daegu, Korea.
- 11Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea.
- 12Division of Hematology-Oncology, Department of Internal Medicine, Daegu Catholic University Medical Center, Daegu, Korea.
- 13Division of Hematology and Oncology, Department of Internal Medicine, Ewha Woman's University School of Medicine, Seoul, Korea.
- 14Department of Hematology/Oncology, Chungnam National University Hospital, Daejeon, Korea.
- 15Department of Hematology-Oncology, Busan National Cancer Center, Pusan National University Hospital Medical Research Institute, Busan, Korea. hemonhs@gmail.com
- KMID: 2158005
- DOI: http://doi.org/10.3346/jkms.2013.28.1.80
Abstract
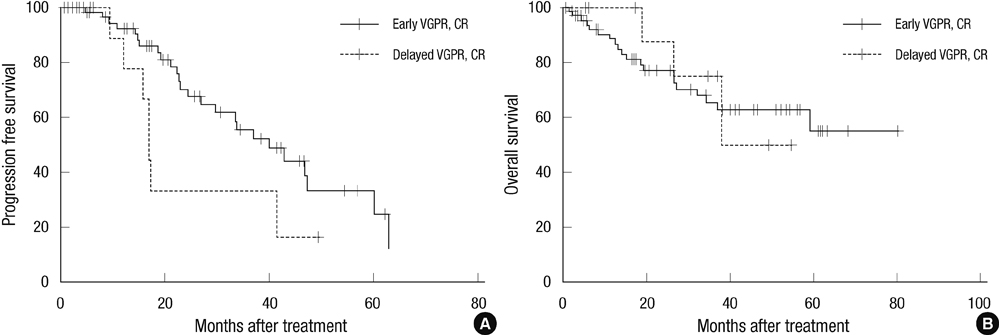
- Novel agents to treat multiple myeloma (MM) have increased complete respone (CR) rates compared with conventional chemotherapy, and the quality of the response to treatment has been correlated with survival. The purpose of our study was to show how of early response to bortezomib combined chemotherapy influences survival in patients with newly diagnosed MM who are ineligible for stem cell transplantation. We assessed patient responses to at least four cycles of bortezomib using the International Myeloma Working Group response criteria. The endpoints were comparisons of progression free survival (PFS) and overall survival (OS) between early good response group (A group) and poor response group (B group). We retrospectively analyzed data from 129 patients registered by the Korean Multiple Myeloma Working Party, a nationwide registration of MM patients. The 3 yr PFS for the A and B groups was 55.6% and 18.4%, respectively (P < 0.001). The 3 yr OS for the A and B groups was 65.3% and 52.9%, respectively (P = 0.078). The early response to at least four cycle of bortezomib before next chemotherapy may help predict PFS in patients with MM who are ineligible stem cell transplantation.
Keyword
MeSH Terms
-
Aged
Antineoplastic Agents/*therapeutic use
Boronic Acids/*therapeutic use
Disease-Free Survival
drugs Therapy, Combination
Female
Humans
Male
Middle Aged
Multiple Myeloma/*drug therapy/mortality
Predictive Value of Tests
Pyrazines/*therapeutic use
Registries
Retrospective Studies
*Stem Cell Transplantation
Treatment Outcome
Antineoplastic Agents
Boronic Acids
Pyrazines
Figure
Reference
-
1. Facon T, Mary JY, Pegourie B, Attal M, Renaud M, Sadoun A, Voillat L, Dorvaux V, Hulin C, Lepeu G, et al. Dexamethasone-based regimens versus melphalan-prednisone for elderly multiple myeloma patients ineligible for high-dose therapy. Blood. 2006. 107:1292–1298.2. Hernandez JM, Garcia-Sanz R, Golvano E, Blade J, Fernandez-Calvo J, Trujillo J, Soler JA, Gardella S, Carbonell F, Mateo G, et al. Randomized comparison of dexamethasone combined with melphalan versus melphalan with prednisone in the treatment of elderly patients with multiple myeloma. Br J Haematol. 2004. 127:159–164.3. Alexanian R, Barlogie B, Tucker S. VAD-based regimens as primary treatment for multiple myeloma. Am J Hematol. 1990. 33:86–89.4. Segeren CM, Sonneveld P, vanderHolt B, Baars JW, Biesma DH, Cornellissen JJ, Croockewit AJ, Dekker AW, Fibbe WE, Löwenberg B, et al. Vincristine, doxorubicin and dexamethasone (VAD) administered as rapid intravenous infusion for first-line treatment in untreated multiple myeloma. Br J Haematol. 1999. 105:127–130.5. Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, Brown J, Drayson MT, Selby PJ. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003. 348:1875–1883.6. Lenhoff S, Hjorth M, Holmberg E, Turesson I, Westin J, Nielsen JL, Wisloff F, Brinch L, Carlson K, Carlsson M, et al. Impact on survival of high-dose therapy with autologous stem cell support in patients younger than 60 years with newly diagnosed multiple myeloma: a population-based study. Blood. 2000. 95:7–11.7. Richardson PG, Mitsiades C, Schlossman R, Munshi N, Anderson K. New drugs for myeloma. Oncologist. 2007. 12:664–689.8. Richardson PG, Mitsiades C, Schlossman R, Ghobrial I, Hideshima T, Munshi N, Anderson KC. Bortezomib in the front-line treatment of multiple myeloma. Expert Rev Anticancer Ther. 2008. 8:1053–1072.9. Gertz MA, Kumar S, Lacy MQ, Dispenzieri A, Dingli D, Hayman SR, Buadi FK, Hogan WJ. Stem cell transplantation in multiple myeloma: impact of response failure with thalidomide or lenalidomide induction. Blood. 2010. 115:2348–2353.10. Kim JS, Kim K, Cheong JW, Min YH, Suh C, Kim H, Jo DY, Ryoo HM, Yoon SS, Lee JH. Complete remission status before autologous stem cell transplantation is an important prognostic factor in patients with multiple myeloma undergoing upfront single autologous transplantation. Biol Blood Marrow Transplant. 2009. 15:463–470.11. Kyle RA, Leong T, Li S, Oken MM, Kay NE, VanNess B, Greipp PR. Complete response in multiple myeloma: clinical trial E9486, an Eastern Cooperative Oncology Group study not involving stem cell transplantation. Cancer. 2006. 106:1958–1966.12. Offidani M, Corvatta L, Piersantelli MN, Visani G, Alesiani F, Brunori M, Galieni P, Catarini M, Burattini M, Centurioni R, et al. Thalidomide, dexamethasone, and pegylated liposomal doxorubicin (ThaDD) for patients older than 65 years with newly diagnosed multiple myeloma. Blood. 2006. 108:2159–2164.13. Palumbo A, Bringhen S, Liberati AM, Caravita T, Falcone A, Callea V, Montanaro M, Ria R, Capaldi A, Zambello R, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. Blood. 2008. 112:3107–3114.14. Hulin C, Facon T, Rodon P, Pegourie B, Benboubker L, Doyen C, Dib M, Guillerm G, Salles B, Eschard JP, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. J Clin Oncol. 2009. 27:3664–3670.15. Ross DM, To LB, Horvath N. Assessment of early paraprotein response to vincristine-doxorubicin-dexamethasone chemotherapy may help guide therapy in multiple myeloma. Intern Med J. 2004. 34:576–578.16. Palumbo A. Early response predicts myeloma outcome. Blood. 2010. 115:2332–2333.17. Schaar CG, Kluin-Nelemans JC, le Cessie S, Franck PF, te Marvelde MC, Wijermans PW. Early response to therapy and survival in multiple myeloma. Br J Haematol. 2004. 125:162–166.18. Ludwig H, Hajek R, Tothova E, Drach J, Adam Z, Labar B, Egyed M, Spicka I, Gisslinger H, Greil R, et al. Thalidomide-dexamethasone compared with melphalan-prednisolone in elderly patients with multiple myeloma. Blood. 2009. 113:3435–3442.19. Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Pegourie B, Renaud M, Harousseau JL, Guillerm G, Chaleteix C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007. 370:1209–1218.20. San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A, Samoilova OS, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008. 359:906–917.21. Shah J, Blade J, Sonneveld P, Harousseau JL, Lantz K, Londhe A, Lowery C, Orlowski RZ. Rapid early monoclonal protein reduction after therapy with bortezomib or bortezomib and pegylated liposomal doxorubicin in relapsed/refractory myeloma is associated with a longer time to progression. Cancer. 2011. 117:3758–3762.22. Palumbo A, Sezer O, Kyle R, Miguel JS, Orlowski RZ, Moreau P, Niesvizky R, Morgan G, Comenzo R, Sonneveld P, et al. International Myeloma Working Group guidelines for the management of multiple myeloma patients ineligible for standard high-dose chemotherapy with autologous stem cell transplantation. Leukemia. 2009. 23:1716–1730.23. Harousseau JL, Palumbo A, Richardson PG, Schlag R, Dimopoulos MA, Shpilberg O, Kropff M, Kentos A, Cavo M, Golenkov A, et al. Superior outcomes associated with complete response in newly diagnosed multiple myeloma patients treated with nonintensive therapy: analysis of the phase 3 VISTA study of bortezomib plus melphalan-prednisone versus melphalan-prednisone. Blood. 2010. 116:3743–3750.24. Palumbo A, Falco P, Corradini P, Falcone A, DiRaimondo F, Giuliani N, Crippa C, Ciccone G, Omedè P, Ambrosini MT, et al. Melphalan, prednisone, and lenalidomide treatment for newly diagnosed myeloma: a report from the GIMEMA-Italian Multiple Myeloma Network. J Clin Oncol. 2007. 25:4459–4465.25. Gay F, Vincent Rajkumar S, Falco P, Kumar S, Dispenzieri A, Petrucci MT, Gertz MA, Boccadoro M, Keith Stewart A, Palumbo A. Lenalidomide plus dexamethasone vs lenalidomide plus melphalan and prednisone: a retrospective study in newly diagnosed elderly myeloma. Eur J Haematol. 2010. 85:200–208.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Outcomes of bortezomib combination chemotherapies in autologous stem cell transplantation-ineligible patients with AL amyloidosis
- Optimal maintenance and consolidation therapy for multiple myeloma in actual clinical practice
- Diagnosis and therapy of multiple myeloma
- Bortezomib and melphalan as a conditioning regimen for autologous stem cell transplantation in multiple myeloma
- A Case of Drug-Induced Hepatitis due to Bortezomib in Multiple Myeloma