Korean J Hematol.
2010 Sep;45(3):183-187. 10.5045/kjh.2010.45.3.183.
Bortezomib and melphalan as a conditioning regimen for autologous stem cell transplantation in multiple myeloma
- Affiliations
-
- 1Department of Internal Medicine, Korea University Medical Center, Seoul, Korea.
- 2Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. kstwoh@skku.edu
- KMID: 2252060
- DOI: http://doi.org/10.5045/kjh.2010.45.3.183
Abstract
- BACKGROUND
High-dose melphalan (200 mg/m2) with autologous stem cell transplantation (ASCT) is the standard treatment for young patients with multiple myeloma (MM). However, the response rates after ASCT are often unsatisfactory. We performed a pilot study by using bortezomib-melphalan as conditioning regimen for ASCT in Korean patients with MM.
METHODS
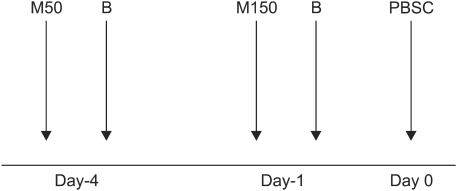
The conditioning regimen consisted of administration of intravenous infusion of bortezomib 1.0 mg/m2 on days -4 and -1 and melphalan 50 mg/m2 (day -4) and 150 mg/m2 (day -1). In this study, we enrolled 6 newly diagnosed patients and 2 patients with relapse.
RESULTS
The disease status of the 6 newly diagnosed patients at ASCT was as follows: 1 complete remission (CR), 1 very good partial remission (VGPR), and 4 partial remissions (PRs). The disease status of the 2 relapsed patients at ASCT was PR. All patients except 1 showed adequate hematologic recovery after ASCT. The median time for the absolute neutrophil counts to increase over 500/mm3 was 13 days (range, 10-19 days). Six patients with VGPR or PR at the time of transplantation showed an improvement in response to CR after ASCT. The patients were followed up without any maintenance treatment after ASCT except 1 patient who died during ASCT. During the follow-up period, CR was maintained in 3 newly diagnosed patients, but the other 4 patients, including 2 newly diagnosed patients, relapsed.
CONCLUSION
Conditioning regimen consisting of bortezomib and melphalan may be effective for ASCT in MM; however, the feasibility of this regimen should be further evaluated in large study populations.
Keyword
MeSH Terms
Figure
Reference
-
1. Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003; 348:1875–1883. PMID: 12736280.
Article2. Palumbo A, Triolo S, Argentino C, et al. Dose-intensive melphalan with stem cell support (MEL100) is superior to standard treatment in elderly myeloma patients. Blood. 1999; 94:1248–1253. PMID: 10438712.
Article3. Moreau P, Facon T, Attal M, et al. Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myélome 9502 randomized trial. Blood. 2002; 99:731–735. PMID: 11806971.4. Desikan KR, Tricot G, Dhodapkar M, et al. Melphalan plus total body irradiation (MEL-TBI) or cyclophosphamide (MEL-CY) as a conditioning regimen with second autotransplant in responding patients with myeloma is inferior compared to historical controls receiving tandem transplants with melphalan alone. Bone Marrow Transplant. 2000; 25:483–487. PMID: 10713623.
Article5. Einsele H, Bamberg M, Budach W, et al. A new conditioning regimen involving total marrow irradiation, busulfan and cyclophosphamide followed by autologous PBSCT in patients with advanced multiple myeloma. Bone Marrow Transplant. 2003; 32:593–599. PMID: 12953132.
Article6. Anagnostopoulos A, Aleman A, Ayers G, et al. Comparison of high-dose melphalan with a more intensive regimen of thiotepa, busulfan, and cyclophosphamide for patients with multiple myeloma. Cancer. 2004; 100:2607–2612. PMID: 15197803.
Article7. Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003; 348:2609–2617. PMID: 12826635.
Article8. Jagannath S, Barlogie B, Berenson J, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004; 127:165–172. PMID: 15461622.
Article9. Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005; 352:2487–2498. PMID: 15958804.
Article10. Mitsiades N, Mitsiades CS, Richardson PG, et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003; 101:2377–2380. PMID: 12424198.
Article11. Berenson JR, Yang HH, Sadler K, et al. Phase I/II trial assessing bortezomib and melphalan combination therapy for the treatment of patients with relapsed or refractory multiple myeloma. J Clin Oncol. 2006; 24:937–944. PMID: 16418495.
Article12. San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008; 359:906–917. PMID: 18753647.
Article13. Palumbo A, Avonto I, Bruno B, et al. Intermediate-dose melphalan (100 mg/m2)/bortezomib/thalidomide/dexamethasone and stem cell support in patients with refractory or relapsed myeloma. Clin Lymphoma Myeloma. 2006; 6:475–477. PMID: 16796778.14. Roussel M, Moreau P, Huynh A, et al. Bortezomib and high-dose melphalan as conditioning regimen before autologous stem cell transplantation in patients with de novo multiple myeloma: a phase 2 study of the Intergroupe Francophone du Myelome (IFM). Blood. 2010; 115:32–37. PMID: 19884643.15. Attal M, Harousseau JL, Facon T, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003; 349:2495–2502. PMID: 14695409.
Article16. Cavo M, Tosi P, Zamagni E, et al. Prospective, randomized study of single compared with double autologous stem-cell transplantation for multiple myeloma: Bologna 96 clinical study. J Clin Oncol. 2007; 25:2434–2441. PMID: 17485707.
Article17. Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004; 4:349–360. PMID: 15122206.
Article18. Rajkumar SV, Richardson PG, Hideshima T, Anderson KC. Proteasome inhibition as a novel therapeutic target in human cancer. J Clin Oncol. 2005; 23:630–639. PMID: 15659509.
Article19. Podar K, Gouill SL, Zhang J, et al. A pivotal role for Mcl-1 in Bortezomib-induced apoptosis. Oncogene. 2008; 27:721–731. PMID: 17653083.
Article20. Chew E, Filshie R, Wei A. Development of fatal bortezomib induced acute lung injury despite concurrent therapy with high-dose dexamethasone. Leuk Lymphoma. 2007; 48:212–213. PMID: 17325874.
Article21. Gotoh A, Ohyashiki K, Oshimi K, et al. Lung injury associated with bortezomib therapy in relapsed/refractory multiple myeloma in Japan: a questionnaire-based report from the "lung injury by bortezomib" joint committee of the Japanese society of hematology and the Japanese society of clinical hematology. Int J Hematol. 2006; 84:406–412. PMID: 17189220.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Busulfan plus melphalan versus high-dose melphalan as conditioning regimens in autologous stem cell transplantation for newly diagnosed multiple myeloma
- Pre-transplant Disease Status is Important for an Improved Outcome of the Second Stem Cell Transplantation in the Myeloma Patients Receiving the First Autologous Stem Cell Transplantation
- Complete Remission in a Patient with Multiple Myeloma after High Dose Melphalan and CD34+ Selected Autologous Transplantation
- Efficacy and Safety of Melphalan, Cyclophosphamide and Dexamethasone (MCD) as a Salvage Treatment for Patients with Relapsed/Refractory Multiple Myeloma
- A Case of Drug-Induced Hepatitis due to Bortezomib in Multiple Myeloma