Blood Res.
2018 Jun;53(2):105-109. 10.5045/br.2018.53.2.105.
Busulfan plus melphalan versus high-dose melphalan as conditioning regimens in autologous stem cell transplantation for newly diagnosed multiple myeloma
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Boramae Medical Center, Seoul, Korea.
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Korea. go01@snu.ac.kr
- 3National Evidence-Based Healthcare Collaborating Agency, Seoul, Korea.
- KMID: 2451027
- DOI: http://doi.org/10.5045/br.2018.53.2.105
Abstract
- BACKGROUND
High-dose melphalan (HDMEL) represents the standard conditioning regimen before autologous stem cell transplant (ASCT) in multiple myeloma (MM), but recent updates have suggested combination of melphalan with bulsulfan (BUMEL) is also associated with favorable outcomes. We performed the current study to address the lack of comparative studies between the two conditioning regimens in Asian populations.
METHODS
Using the Korean National Health Insurance and Korean Health Insurance Review and Assessment Service databases, 1,304 patients newly diagnosed with MM undergoing ASCT between January 2010 and December 2014 were identified. Patients were divided according to conditioning regimen (HDMEL vs. BUMEL), and after case matching, 428 patients undergoing HDMEL conditioning were compared to 107 patients undergoing BUMEL conditioning with respect to clinical course and treatment outcomes.
RESULTS
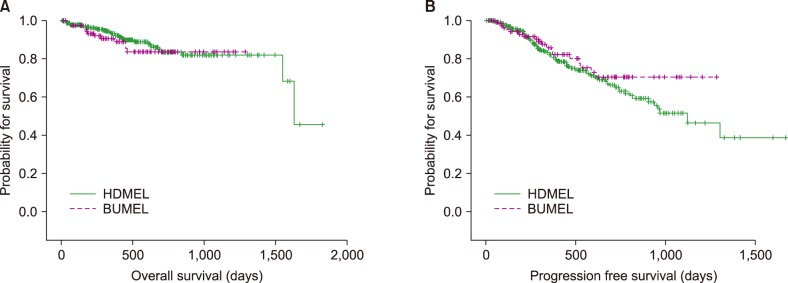
The 3-year progression-free survival (PFS) was 52.5% for the HDMEL conditioning group versus 70.3% for the BUMEL conditioning group (P=0.043). The 3-year overall survival (OS) was 82.0% versus 83.5% (P=0.525), respectively. Although not statistically significant, BUMEL conditioning was associated with more platelet transfusion, while HDMEL was associated with more granulocyte colony stimulating factor support. In multivariate analysis, BUMEL conditioning was not inferior to HDMEL conditioning in regard to both PFS and OS.
CONCLUSION
Our study confirmed that BUMEL is an effective and well-tolerated alternative to HDMEL conditioning, with better PFS.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Development and validation of a comorbidity index for predicting survival outcomes after allogeneic stem cell transplantation in adult patients with acute leukemia: a Korean nationwide cohort study
Sung-Soo Park, Hee-Je Kim, Tong Yoon Kim, Joon yeop Lee, Jong Hyuk Lee, Gi June Min, Silvia Park, Jae-Ho Yoon, Sung-Eun Lee, Byung-Sik Cho, Ki-Seong Eom, Yoo-Jin Kim, Seok Lee, Dong-Wook Kim
Blood Res. 2021;56(3):184-196. doi: 10.5045/br.2021.2021107.
Reference
-
1. Djulbegovic B, Kumar A. Multiple myeloma: detecting the effects of new treatments. Lancet. 2008; 371:1642–1644. PMID: 18486726.
Article2. Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016; 91:719–734. PMID: 27291302.
Article3. Maybury B, Cook G, Pratt G, Yong K, Ramasamy K. Augmenting autologous stem cell transplantation to improve outcomes in myeloma. Biol Blood Marrow Transplant. 2016; 22:1926–1937. PMID: 27288955.
Article4. Raza S, Safyan RA, Rosenbaum E, Bowman AS, Lentzsch S. Optimizing current and emerging therapies in multiple myeloma: a guide for the hematologist. Ther Adv Hematol. 2017; 8:55–70. PMID: 28203342.
Article5. Reece D, Song K, LeBlanc R, et al. Efficacy and safety of busulfan-based conditioning regimens for multiple myeloma. Oncologist. 2013; 18:611–618. PMID: 23628980.
Article6. Gay F, Oliva S, Petrucci MT, et al. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol. 2015; 16:1617–1629. PMID: 26596670.
Article7. Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014; 371:895–905. PMID: 25184862.
Article8. Sengsayadeth S, Malard F, Savani BN, Garderet L, Mohty M. Posttransplant maintenance therapy in multiple myeloma: the changing landscape. Blood Cancer J. 2017; 7:e545. PMID: 28338672.
Article9. Fritz E, Ludwig H. Interferon-alpha treatment in multiple myeloma: meta-analysis of 30 randomised trials among 3948 patients. Ann Oncol. 2000; 11:1427–1436. PMID: 11142483.10. Shustik C, Belch A, Robinson S, et al. A randomised comparison of melphalan with prednisone or dexamethasone as induction therapy and dexamethasone or observation as maintenance therapy in multiple myeloma: NCIC CTG MY.7. Br J Haematol. 2007; 136:203–211. PMID: 17233817.
Article11. Ludwig H, Durie BG, McCarthy P, et al. IMWG consensus on maintenance therapy in multiple myeloma. Blood. 2012; 119:3003–3015. PMID: 22271445.
Article12. McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012; 366:1770–1781. PMID: 22571201.13. Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012; 366:1782–1791. PMID: 22571202.
Article14. Moreau P, Facon T, Attal M, et al. Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myélome 9502 randomized trial. Blood. 2002; 99:731–735. PMID: 11806971.15. Lahuerta JJ, Martinez-Lopez J, Grande C, et al. Conditioning regimens in autologous stem cell transplantation for multiple myeloma: a comparative study of efficacy and toxicity from the Spanish Registry for Transplantation in Multiple Myeloma. Br J Haematol. 2000; 109:138–147. PMID: 10848793.
Article16. Lahuerta JJ, Grande C, Blade J, et al. Myeloablative treatments for multiple myeloma: update of a comparative study of different regimens used in patients from the Spanish registry for transplantation in multiple myeloma. Leuk Lymphoma. 2002; 43:67–74. PMID: 11908738.
Article17. Blanes M, Lahuerta JJ, González JD, et al. Intravenous busulfan and melphalan as a conditioning regimen for autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: a matched comparison to a melphalan-only approach. Biol Blood Marrow Transplant. 2013; 19:69–74. PMID: 22897964.
Article18. Lahuerta JJ, Mateos MV, Martínez-López J, et al. Busulfan 12 mg/kg plus melphalan 140 mg/m2 versus melphalan 200 mg/m2 as conditioning regimens for autologous transplantation in newly diagnosed multiple myeloma patients included in the PETHEMA/GEM2000 study. Haematologica. 2010; 95:1913–1920. PMID: 20663944.
Article19. Ria R, Falzetti F, Ballanti S, et al. Melphalan versus melphalan plus busulphan in conditioning to autologous stem cell transplantation for low-risk multiple myeloma. Hematol J. 2004; 5:118–122. PMID: 15048061.
Article20. Kim DS. Introduction: health of the health care system in Korea. Soc Work Public Health. 2010; 25:127–141. PMID: 20391257.
Article21. Kim SD, Lee JH, Hur EH, et al. Influence of GST gene polymorphisms on the clearance of intravenous busulfan in adult patients undergoing hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011; 17:1222–1230. PMID: 21215809.
Article22. Chiesa R, Cappelli B, Crocchiolo R, et al. Unpredictability of intravenous busulfan pharmacokinetics in children undergoing hematopoietic stem cell transplantation for advanced beta thalassemia: limited toxicity with a dose-adjustment policy. Biol Blood Marrow Transplant. 2010; 16:622–628. PMID: 19963071.
Article23. Hahn T, Zhelnova E, Sucheston L, et al. A deletion polymorphism in glutathione-S-transferase mu (GSTM1) and/or theta (GSTT1) is associated with an increased risk of toxicity after autologous blood and marrow transplantation. Biol Blood Marrow Transplant. 2010; 16:801–808. PMID: 20074657.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bortezomib and melphalan as a conditioning regimen for autologous stem cell transplantation in multiple myeloma
- Complete Remission in a Patient with Multiple Myeloma after High Dose Melphalan and CD34+ Selected Autologous Transplantation
- Pre-transplant Disease Status is Important for an Improved Outcome of the Second Stem Cell Transplantation in the Myeloma Patients Receiving the First Autologous Stem Cell Transplantation
- Pharmacokinetics of High-dose Intravenous Melphalan in Patients Undergoing Autologous Stem Cell Transplantation
- Efficacy and Safety of Melphalan, Cyclophosphamide and Dexamethasone (MCD) as a Salvage Treatment for Patients with Relapsed/Refractory Multiple Myeloma