Chonnam Med J.
2019 Jan;55(1):25-30. 10.4068/cmj.2019.55.1.25.
Efficacy and Safety of Melphalan, Cyclophosphamide and Dexamethasone (MCD) as a Salvage Treatment for Patients with Relapsed/Refractory Multiple Myeloma
- Affiliations
-
- 1Department of Hematology-Oncology, Wonkwang University Hospital, Iksan, Korea.
- 2Department of Hematology-Oncology, Chonnam National University Hwasun Hospital, Hwasun, Korea. drjejung@chonnam.ac.kr
- KMID: 2432252
- DOI: http://doi.org/10.4068/cmj.2019.55.1.25
Abstract
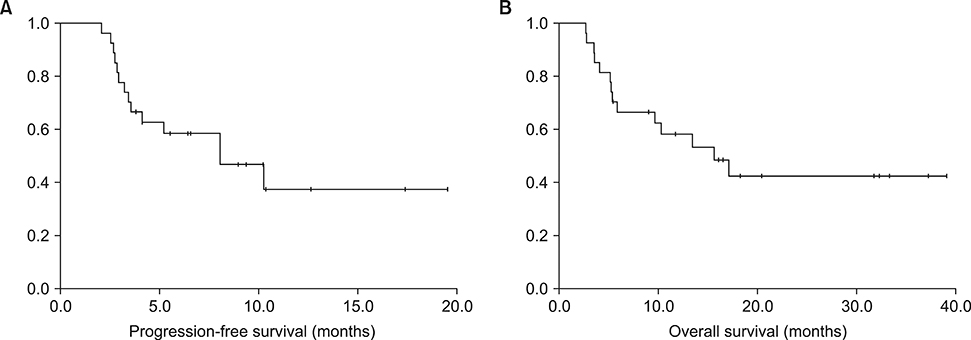
- This study investigated the efficacy and safety of melphalan, cyclophosphamide, and dexamethasone (MCD) as a salvage regimen for heavily treated relapsed or refractory multiple myeloma patients. We retrospectively analyzed a total of 27 patients who received the MCD regimen between April 2011 and November 2013. The MCD regimen consisted of oral melphalan 6.75 mg/m² on days 1-4, once-weekly dose of oral cyclophosphamide 300 mg/m2 and dexamethasone 20 mg/m² on days 1-4 and days 15-18. Each cycle was repeated every 28 days. The median age of the patients was 66 years and the MCD regimen was initiated at a median 37.7 months from diagnosis. Patients received a median of five regimens including autologous stem cell transplantation. The overall response rate was 25.9% (very good partial response 3.7%, partial response 22.2%) and 8 (29.6%) patients achieved a minor response. Median progression-free survival was 5.6 months (95% confidence interval [CI], 4.2-8.5) ; overall survival 11.7 months (95% CI, 5.4-16.6). Grade 3 or 4 neutropenia and thrombocytopenia were observed in 51.8% and 33.3%, respectively. Although the overall response rate is relatively low, the MCD regimen may have a role as a bridge to a novel regimen in heavily pretreated patients with MM.
Keyword
MeSH Terms
Figure
Reference
-
1. Dimopoulos M, Spencer A, Attal M, Prince HM, Harousseau JL, Dmoszynska A, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007; 357:2123–2132.
Article2. Anderson KC. Progress and paradigms in multiple myeloma. Clin Cancer Res. 2016; 22:5419–5427.
Article3. Zago M, Oehrlein K, Rendl C, Hahn-Ast C, Kanz L, Weisel K. Lenalidomide in relapsed and refractory multiple myeloma disease: feasibility and benefits of long-term treatment. Ann Hematol. 2014; 93:1993–1999.
Article4. Baz RC, Martin TG 3rd, Lin HY, Zhao X, Shain KH, Cho HJ, et al. Randomized multicenter phase 2 study of pomalidomide, cyclophosphamide, and dexamethasone in relapsed refractory myeloma. Blood. 2016; 127:2561–2568.
Article5. Usmani SZ, Weiss BM, Plesner T, Bahlis NJ, Belch A, Lonial S, et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood. 2016; 128:37–44.
Article6. Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014; 371:895–905.
Article7. Song MK, Chung JS, Shin HJ, Moon JH, Lee JJ, Yoon SS, et al. Cyclophosphamide-containing regimen (TCD) is superior to melphalan-containing regimen (MPT) in elderly multiple myeloma patients with renal impairment. Ann Hematol. 2012; 91:889–896.
Article8. Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005; 105:2862–2868.
Article9. García-Sanz R, González-Fraile MI, Sierra M, López C, González M, San Miguel JF. The combination of thalidomide, cyclophosphamide and dexamethasone (ThaCyDex) is feasible and can be an option for relapsed/refractory multiple myeloma. Hematol J. 2002; 3:43–48.
Article10. Kropff M, Bisping G, Schuck E, Liebisch P, Lang N, Hentrich M, et al. Bortezomib in combination with intermediate-dose dexamethasone and continuous low-dose oral cyclophosphamide for relapsed multiple myeloma. Br J Haematol. 2007; 138:330–337.
Article11. Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016; 17:e328–e346.
Article12. Engelhardt M, Terpos E, Kleber M, Gay F, Wäsch R, Morgan G, et al. European Myeloma Network recommendations on the evaluation and treatment of newly diagnosed patients with multiple myeloma. Haematologica. 2014; 99:232–242.
Article13. Laubach J, Garderet L, Mahindra A, Gahrton G, Caers J, Sezer O, et al. Management of relapsed multiple myeloma: recommendations of the International Myeloma Working Group. Leukemia. 2016; 30:1005–1017.
Article14. Vincent Rajkumar S. Multiple myeloma: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol. 2014; 89:999–1009.15. National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Multiple Myeloma Version 1. 2019. p. 1–78.16. Griffin PT, Ho VQ, Fulp W, Nishihori T, Shain KH, Alsina M, et al. A comparison of salvage infusional chemotherapy regimens for recurrent/refractory multiple myeloma. Cancer. 2015; 121:3622–3630.
Article17. Lee CK, Barlogie B, Munshi N, Zangari M, Fassas A, Jacobson J, et al. DTPACE: an effective, novel combination chemotherapy with thalidomide for previously treated patients with myeloma. J Clin Oncol. 2003; 21:2732–2739.
Article18. Gerrie AS, Mikhael JR, Cheng L, Jiang H, Kukreti V, Panzarella T, et al. D(T)PACE as salvage therapy for aggressive or refractory multiple myeloma. Br J Haematol. 2013; 161:802–810.
Article19. Dadacaridou M, Papanicolaou X, Maltesas D, Megalakaki C, Patos P, Panteli K, et al. Dexamethasone, cyclophosphamide, etoposide and cisplatin (DCEP) for relapsed or refractory multiple myeloma patients. J BUON. 2007; 12:41–44.20. Park S, Lee SJ, Jung CW, Jang JH, Kim SJ, Kim WS, et al. DCEP for relapsed or refractory multiple myeloma after therapy with novel agents. Ann Hematol. 2014; 93:99–105.
Article21. Kim SJ, Bang SM, Choi YS, Jo DY, Kim JS, Lee H, et al. Bendamustine in heavily pre-treated multiple myeloma patients: results of a retrospective analysis from the Korean Multiple Myeloma Working Party. Blood Res. 2016; 51:193–199.
Article22. Damaj G, Malard F, Hulin C, Caillot D, Garidi R, Royer B, et al. Efficacy of bendamustine in relapsed/refractory myeloma patients: results from the French compassionate use program. Leuk Lymphoma. 2012; 53:632–634.
Article23. Leoni LM, Bailey B, Reifert J, Bendall HH, Zeller RW, Corbeil J, et al. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res. 2008; 14:309–317.
Article24. Gentile M, Vigna E, Recchia AG, Morabito L, Mendicino F, Giagnuolo G, et al. Bendamustine in multiple myeloma. Eur J Haematol. 2015; 95:377–388.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Cyclophosphamide and Prednisone as a First-line Treatment for Non-transplant Candidates with Multiple Myeloma
- Treatment of relapsed and refractory multiple myeloma
- Myelomatous effusion with poor response to chemotherapy
- Salvage Therapy with Thalidomide in Patients with Relapsed or Refractory Multiple Myeloma
- Bortezomib-Based Salvage Chemotherapy in Refractory/Relapsed Multiple Myeloma Patients: A Single Center Experience in Real Clinical Practice