Immune Netw.
2012 Jun;12(3):126-128. 10.4110/in.2012.12.3.126.
A Case of Drug-Induced Hepatitis due to Bortezomib in Multiple Myeloma
- Affiliations
-
- 1Division of Hematology, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul 137-701, Korea. ckmin@catholic.ac.kr
- 2Department of Hospital Pathology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul 137-701, Korea.
- KMID: 2168011
- DOI: http://doi.org/10.4110/in.2012.12.3.126
Abstract
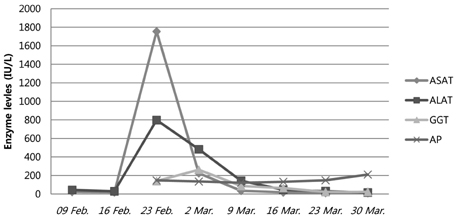
- We report on a case of severe hepatotoxicity in a 52-year-old male with multiple myeloma (MM) who had received bortezomib therapy. At patient presentation, liver enzymes were normal, but started to markedly increase 3 days after the patient's second dose of bortezomib was administered, when free kappa light chains were noticeably reduced in the serum. After discontinuation of bortezomib, liver enzymes recovered gradually to baseline. Then, the patient was started on a thalidomide-containing regimen, which he was able to tolerate well. The patient achieved complete remission prior to autologous stem cell transplantation (ASCT). The patient underwent ASCT without occurrence of further liver toxicity.
Keyword
MeSH Terms
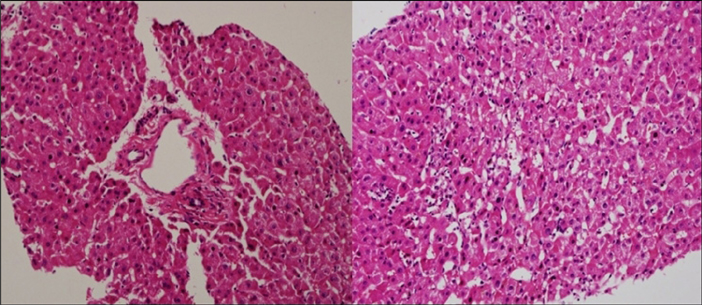
Figure
Reference
-
1. Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S, Elliott PJ. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999. 59:2615–2622.2. Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, Anderson KC. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001. 61:3071–3076.3. Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina M, Alexanian R, Siegel D, Orlowski RZ, Kuter D, Limentani SA, Lee S, Hideshima T, Esseltine DL, Kauffman M, Adams J, Schenkein DP, Anderson KC. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003. 348:2609–2617.
Article4. Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002. 3:221–227.5. Rosiñol L, Montoto S, Cibeira MT, Bladé J. Bortezomib-induced severe hepatitis in multiple myeloma: a case report. Arch Intern Med. 2005. 165:464–465.
Article6. Ghobrial IM, Rajkumar SV. Management of thalidomide toxicity. J Support Oncol. 2003. 1:194–205.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Acute Pancreatitis Caused by Bortezomib in a Patient with Multiple Myeloma
- Two Cases of Bortezomib-induced Drug Eruption Presenting as Multiple Plaques on the Trunk
- Bortezomib-induced Histiocytoid Sweet Syndrome
- Reversible Heart Failure after Bortezomib Treatment in a Patient with Multiple Myeloma
- Complete Atrioventricular Block Secondary to Bortezomib Use in Multiple Myeloma