Yonsei Med J.
2011 Jan;52(1):196-198. 10.3349/ymj.2011.52.1.196.
Complete Atrioventricular Block Secondary to Bortezomib Use in Multiple Myeloma
- Affiliations
-
- 1Department of Internal Medicine, Inha University College of Medicine, Incheon, Korea. kdhmd@inha.ac.kr
- KMID: 1106457
- DOI: http://doi.org/10.3349/ymj.2011.52.1.196
Abstract
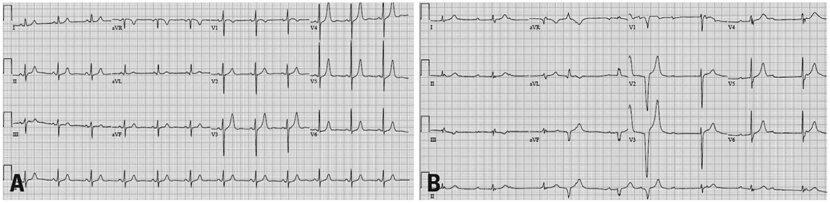
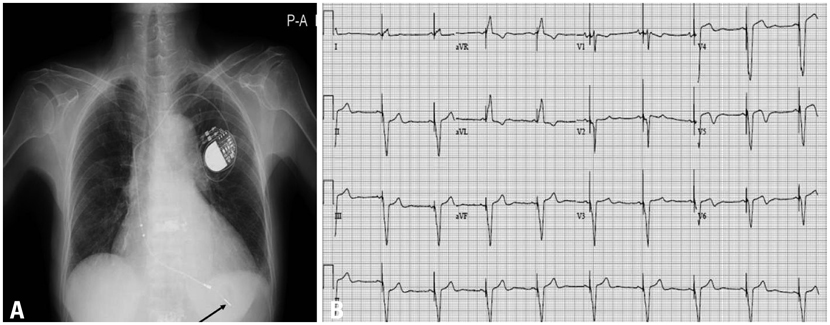
- Bortezomib is an inhibitor of 26S proteasome, which is an effective treatment for multiple myeloma. The common adverse effects of bortezomib are asthenic conditions, gastrointestinal disturbances, and peripheral neuropathy. Here we describe a patient with dyspnea and general weakness because of complete atrioventricular block while receiving bortezomib. We immediately stopped bortezomib, and after inserting a permanent VDD pacemaker, the patients' symptoms disappeared.
MeSH Terms
Figure
Reference
-
1. Dasanu CA. Complete heart block secondary to bortezomib use in multiple myeloma. J Oncol Pharm Pract. 2010. 04. 20. [Epub ahead of print].
Article2. Colson K, Doss DS, Swift R, Tariman J, Thomas TE. Bortezomib, a newly approved proteasome inhibitor for the treatment of multiple myeloma: nursing implications. Clin J Oncol Nurs. 2004. 8:473–480.
Article3. Cavaletti G, Gilardini A, Canta A, Rigamonti L, Rodriguez-Menendez V, Ceresa C, et al. Bortezomib-induced peripheral neurotoxicity: a neurophysiological and pathological study in the rat. Exp Neurol. 2007. 204:317–325.
Article4. Shah MH, Young D, Kindler HL, Webb I, Kleiber B, Wright J, et al. Phase II study of the proteasome inhibitor bortezomib (PS-341) in patients with metastatic neuroendocrine tumors. Clin Cancer Res. 2004. 10:6111–6118.
Article5. Berenson JR, Jagannath S, Barlogie B, Siegel DT, Alexanian R, Richardson PG, et al. Safety of prolonged therapy with bortezomib in relapsed or refractory multiple myeloma. Cancer. 2005. 104:2141–2148.
Article6. Reece DE, Sanchorawala V, Hegenbart U, Merlini G, Palladini G, Fermand JP, et al. Weekly and twice-weekly bortezomib in patients with systemic AL amyloidosis: results of a phase 1 dose-escalation study. Blood. 2009. 114:1489–1497.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Acute Pancreatitis Caused by Bortezomib in a Patient with Multiple Myeloma
- Reversible Heart Failure after Bortezomib Treatment in a Patient with Multiple Myeloma
- Huge Cutaneous Involvement of Multiple Myeloma
- A Case of Cutaneous Plasmacytoma Treated with Bortezomib and Radiotherapy
- Bortezomib-induced Histiocytoid Sweet Syndrome