Blood Res.
2021 Dec;56(4):266-278. 10.5045/br.2021.2021121.
Outcomes of bortezomib combination chemotherapies in autologous stem cell transplantation-ineligible patients with AL amyloidosis
- Affiliations
-
- 1Division of Hematology and Oncology, Department of Internal Medicine, Hanyang University Guri Hospital, Guri, Korea
- 2Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Division of Cardiology, Department of Medicine, Heart Vascular Stroke Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Departments of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 5Departments of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 6Division of Nephrology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 7Department of Nuclear Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2524086
- DOI: http://doi.org/10.5045/br.2021.2021121
Abstract
- Background
Treatment protocols for light chain (AL) amyloidosis have been derived from myeloma treatment. Bortezomib is a key drug used for the treatment of myeloma and AL amyloidosis. We retrospectively investigated the efficacy and toxicity of bortezomib-based chemotherapy in patients with newly diagnosed AL amyloidosis.
Methods
We reviewed the outcomes of newly diagnosed autologous stem cell transplantation (auto-SCT)-ineligible AL amyloidosis patients who received bortezomib-based chemotherapy at a referral center between 2011 and 2017.
Results
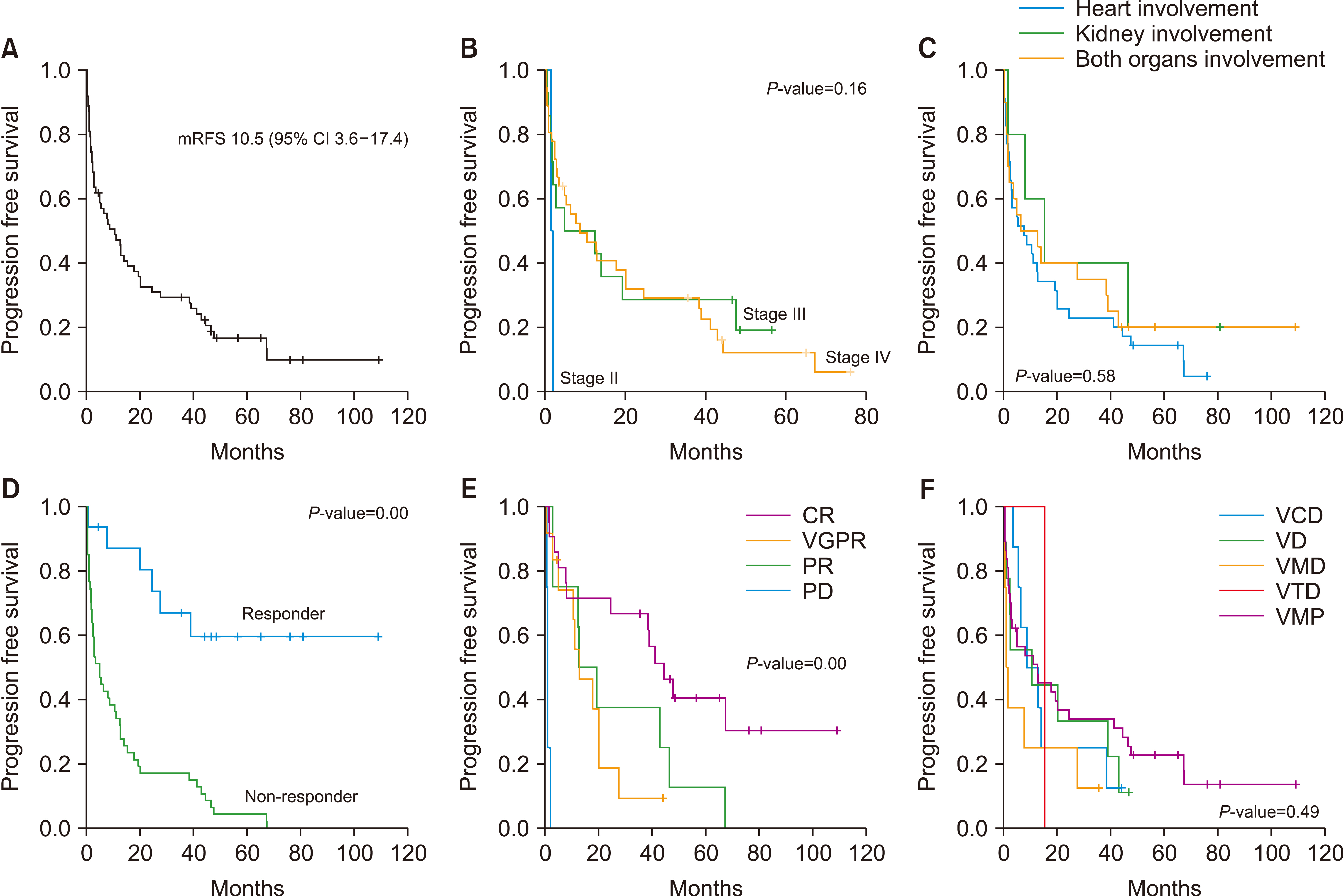
Of 63 patients who received bortezomib-based chemotherapy, 32 were male, and the median age was 66 years (range, 42‒82 yr). The hematologic overall response rate (ORR) was 65.1%, and the chemotherapy regimen with the best hematologic response was VMP (75.7%, 28/37). Sixty patients had significant organ (heart or kidney) involvement; 28.3% of patients (N=17) had major organ responses after chemotherapy. With a median follow-up of 34 months, there was no significant difference in progression-free survival (P =0.49) or overall survival (P =0.67) according to regimen. Most hematologic and non-hematologic problems were manageable.
Conclusion
Various chemotherapy combinations based on bortezomib are currently employed in the clinical setting, but no difference was found in terms of efficacy or toxicity.
Figure
Reference
-
1. Merlini G, Bellotti V. 2003; Molecular mechanisms of amyloidosis. N Engl J Med. 349:583–96. DOI: 10.1056/NEJMra023144. PMID: 12904524.
Article2. Palladini G, Dispenzieri A, Gertz MA, et al. 2012; New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 30:4541–9. DOI: 10.1200/JCO.2011.37.7614. PMID: 23091105.
Article3. Comenzo RL, Reece D, Palladini G, et al. 2012; Consensus guidelines for the conduct and reporting of clinical trials in systemic light-chain amyloidosis. Leukemia. 26:2317–25. DOI: 10.1038/leu.2012.100. PMID: 22475872.
Article4. Duhamel S, Mohty D, Magne J, et al. 2017; Incidence and prevalence of light chain amyloidosis: a population-based study. Blood (ASH Annual Meeting Abstracts). 130(Suppl):5577.5. Dispenzieri A, Gertz MA, Kyle RA, et al. 2004; Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol. 22:3751–7. DOI: 10.1200/JCO.2004.03.029. PMID: 15365071.
Article6. Kumar SK, Gertz MA, Lacy MQ, et al. 2011; Recent improvements in survival in primary systemic amyloidosis and the importance of an early mortality risk score. Mayo Clin Proc. 86:12–8. DOI: 10.4065/mcp.2010.0480. PMID: 21193650. PMCID: PMC3012628.
Article7. Sitia R, Palladini G, Merlini G. 2007; Bortezomib in the treatment of AL amyloidosis: targeted therapy? Haematologica. 92:1302–7. DOI: 10.3324/haematol.12136. PMID: 18024367.
Article8. Palladini G, Merlini G. 2016; What is new in diagnosis and management of light chain amyloidosis? Blood. 128:159–68. DOI: 10.1182/blood-2016-01-629790. PMID: 27053535.
Article9. Al Hamed R, Bazarbachi AH, Malard F, Harousseau JL, Mohty M. 2019; Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 9:44. DOI: 10.1038/s41408-019-0205-9. PMID: 30962422. PMCID: PMC6453900.
Article10. Sidiqi MH, Aljama MA, Buadi FK, et al. 2018; Stem cell transplantation for light chain amyloidosis: decreased early mortality over time. J Clin Oncol. 36:1323–9. DOI: 10.1200/JCO.2017.76.9554. PMID: 29558277.
Article11. Lee JY, Lim SH, Kim SJ, et al. 2014; Bortezomib, melphalan, and prednisolone combination chemotherapy for newly diagnosed light chain (AL) amyloidosis. Amyloid. 21:261–6. DOI: 10.3109/13506129.2014.960560. PMID: 25248716.
Article12. Mikhael JR, Schuster SR, Jimenez-Zepeda VH, et al. 2012; Cyclophosphamide-bortezomib-dexamethasone (CyBorD) produces rapid and complete hematologic response in patients with AL amyloidosis. Blood. 119:4391–4. DOI: 10.1182/blood-2011-11-390930. PMID: 22331188. PMCID: PMC3557400.
Article13. Kastritis E, Anagnostopoulos A, Roussou M, et al. 2007; Treatment of light chain (AL) amyloidosis with the combination of bortezomib and dexamethasone. Haematologica. 92:1351–8. DOI: 10.3324/haematol.11325. PMID: 18024372.
Article14. Kastritis E, Wechalekar AD, Dimopoulos MA, et al. 2010; Bortezomib with or without dexamethasone in primary systemic (light chain) amyloidosis. J Clin Oncol. 28:1031–7. DOI: 10.1200/JCO.2009.23.8220. PMID: 20085941.
Article15. Palladini G, Milani P, Foli A, et al. 2014; Melphalan and dexamethasone with or without bortezomib in newly diagnosed AL amyloidosis: a matched case-control study on 174 patients. Leukemia. 28:2311–6. DOI: 10.1038/leu.2014.227. PMID: 25059496.
Article16. Kastritis E, Leleu X, Arnulf B, et al. 2020; Bortezomib, melphalan, and dexamethasone for light-chain amyloidosis. J Clin Oncol. 38:3252–60. DOI: 10.1200/JCO.20.01285. PMID: 32730181.
Article17. Kumar S, Dispenzieri A, Lacy MQ, et al. 2012; Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 30:989–95. DOI: 10.1200/JCO.2011.38.5724. PMID: 22331953. PMCID: PMC3675680.
Article18. Huang X, Wang Q, Chen W, Ren G, Liu Z. 2016; Bortezomib with dexamethasone as first-line treatment for AL amyloidosis with renal involvement. Amyloid. 23:51–7. DOI: 10.3109/13506129.2016.1138939. PMID: 26862817.
Article19. Palladini G, Dispenzieri A, Gertz MAA, et al. 2010; Validation of the criteria of response to treatment in al amyloidosis. Blood (ASH Annual Meeting Abstracts). 116(Suppl):1364. DOI: 10.1182/blood.V116.21.1364.1364.
Article20. Wechalekar AD, Gillmore JD, Bird J, et al. 2015; Guidelines on the management of AL amyloidosis. Br J Haematol. 168:186–206. DOI: 10.1111/bjh.13155. PMID: 25303672.
Article21. Dimopoulos MA, Kastritis E. 2011; Bortezomib for AL amyloidosis: moving forward. Blood. 118:827–8. DOI: 10.1182/blood-2011-05-355115. PMID: 21799093.
Article22. Vaxman I, Gertz M. 2019; Recent advances in the diagnosis, risk stratification, and management of systemic light-chain amyloidosis. Acta Haematol. 141:93–106. DOI: 10.1159/000495455. PMID: 30650422.
Article23. Larocca A, Bringhen S, Petrucci MT, et al. 2016; A phase 2 study of three low-dose intensity subcutaneous bortezomib regimens in elderly frail patients with untreated multiple myeloma. Leukemia. 30:1320–6. DOI: 10.1038/leu.2016.36. PMID: 26898189.
Article24. Niesvizky R, Flinn IW, Rifkin R, et al. 2015; Community-based phase IIIB trial of three UPFRONT bortezomib-based myeloma regimens. J Clin Oncol. 33:3921–9. DOI: 10.1200/JCO.2014.58.7618. PMID: 26056177.
Article25. Kastritis E, Gavriatopoulou M, Roussou M, et al. 2017; Addition of cyclophosphamide and higher doses of dexamethasone do not improve outcomes of patients with AL amyloidosis treated with bortezomib. Blood Cancer J. 7:e570. DOI: 10.1038/bcj.2017.47. PMID: 28622303. PMCID: PMC5520394.
Article26. Dumas B, Yameen H, Sarosiek S, Sloan JM, Sanchorawala V. 2020; Presence of t(11;14) in AL amyloidosis as a marker of response when treated with a bortezomib-based regimen. Amyloid. 27:244–9. DOI: 10.1080/13506129.2020.1778461. PMID: 32551974.
Article27. Jun HJ, Kim K, Kim SJ, et al. 2013; Clinical features and treatment outcome of primary systemic light-chain amyloidosis in Korea: results of multicenter analysis. Am J Hematol. 88:52–5. DOI: 10.1002/ajh.23333. PMID: 23027340.
Article28. Wechalekar AD, Offer M, Gillmore JD, Hawkins PN, Lachmann HJ. 2009; Cardiac amyloidosis, a monoclonal gammopathy and a potentially misleading mutation. Nat Clin Pract Cardiovasc Med. 6:128–33. DOI: 10.1038/ncpcardio1423. PMID: 19079367.
Article29. Sanchorawala V, Palladini G, Kukreti V, et al. 2017; A phase 1/2 study of the oral proteasome inhibitor ixazomib in relapsed or refractory AL amyloidosis. Blood. 130:597–605. DOI: 10.1182/blood-2017-03-771220. PMID: 28550039. PMCID: PMC6911836.
Article30. Kastritis E, Dialoupi I, Gavriatopoulou M, et al. 2019; Primary treatment of light-chain amyloidosis with bortezomib, lenalidomide, and dexamethasone. Blood Adv. 3:3002–9. DOI: 10.1182/bloodadvances.2019000147. PMID: 31648323. PMCID: PMC6849948.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Remission of Renal Amyloidosis after Autologous Peripheral Blood Stem Cell Transplantation
- A Case of Scleroderma following Autologous Peripheral Stem Cell Transplantation
- Bortezomib Therapy Followed by Autologous Stem Cell Transplantation in POEMS Syndrome
- AL amyloidosis: advances in diagnosis and management
- Bortezomib and melphalan as a conditioning regimen for autologous stem cell transplantation in multiple myeloma