J Korean Med Sci.
2004 Aug;19(4):591-597. 10.3346/jkms.2004.19.4.591.
Protective Effect of Heat Shock Protein 70 Against Oxidative Stresses in Human Corneal Fibroblasts
- Affiliations
-
- 1Department of Ophthalmology, College of Medicine, Chung-Ang University, Seoul, Korea. jck50ey@kornet.net
- 2Korea Institute of Science and Technology, Seoul, Korea.
- KMID: 2157691
- DOI: http://doi.org/10.3346/jkms.2004.19.4.591
Abstract
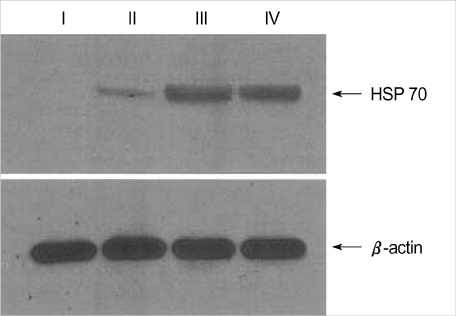
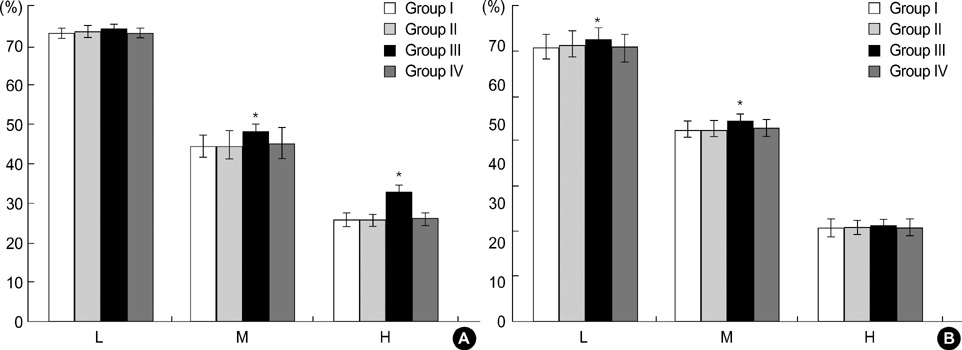
- We evaluated DNA protection effect of heat shock protein (HSP) against cytotoxic effects of exogenous nitric oxide (NO) and reactive oxygen intermediate (ROI). Cultured human corneal fibroblasts were divided into 4 groups. Control (Group I) was not exposed to a sub-lethal heat treatment. Other 3 groups were exposed to 43 degrees C for 1 hr, then incubated at 37 degrees C during different duration (1, 6, 24 hr, Group II, III, IV, respectively). Expression pattern of HSP 70 was analyzed by Western blot. Cell viability was measured by MTT assay and the relationship between HSP 70 expression and DNA damage was examined by terminal deoxyribonucleotidyl transferase mediated dUTP-digoxigenin nick and labeling (TUNEL) stain and single cell gel electrophoresis. Expression pattern of HSP 70 was dependent on recovery times. Cell viability following heat treatment was significantly increased and the TUNEL positive cell number was decreased at 6 hr. In single cell gel electrophoresis, tail moments were increased in a dose-dependent manner by SNAP and X/XO. Following heat treatment, tail moments showed decreased significantly at 6 hr. These results suggest that induction of HSP 70 by sub-lethal heat treatment is closely related with cytoprotective effects against oxidative stresses in human corneal fibroblasts.
Keyword
MeSH Terms
-
Cell Survival
Cells, Cultured
Cornea/*cytology
DNA Damage
Dose-Response Relationship, Drug
Fibroblasts/cytology/drug effects/*metabolism
Heat
Heat-Shock Proteins 70/genetics/*metabolism
Humans
In Situ Nick-End Labeling
Nitric Oxide/metabolism
Nitric Oxide Donors/pharmacology
*Oxidative Stress
Reactive Oxygen Species/metabolism
Research Support, Non-U.S. Gov't
S-Nitroso-N-Acetylpenicillamine/pharmacology
Xanthine/pharmacology
Xanthine Oxidase/pharmacology
Figure
Reference
-
1. Wong HR, Ryan M, Menendez IY, Denenberg A, Wispe JR. Heat shock protein induction protects human respiratory epithelium against nitric oxide-mediated cytotoxicity. Shock. 1997. 8:213–218.
Article2. Jäättela M. Heat shock proteins as cellular lifeguards. Ann Med. 1999. 31:261–271.3. Wong HR, Mannix RJ, Rusnak JM, Boota A, Zar H, Watkins SC, Lazo JS, Pitt BR. The heat shock response attenuates lipopolysaccharide-mediate apoptosis in cultured sheep pulmonary artery endothelial cells. Am J Respir Cell Mol Biol. 1996. 15:745–751.4. Lindquist S, Craig EA. The heat shock proteins. Annu Rev Genet. 1988. 22:631–677.5. Kiang JG, Tsokos GC. Heat shock protein 70kDA: molecular biology, biochemistry, and physiology. Pharmacol Ther. 1998. 80:183–201.6. Tavaria M, Gabriele T, Kola I, Anderson RL. A hitchhiker's guide to human Hsp 70 family. Cell Stress Chaperones. 1996. 1:23–28.7. Hughes CM, McKelvey-Martin VJ, Lewis SEM. Human sperm DNA integrity assessed by the Comet and ELISA assays. Mutagenesis. 1999. 14:71–75.8. Lee JG, Madden MC, Reed W, Adler K, Devlin R. The use of the single cell gel electrophoresis assay in detecting DNA single strand breaks in lung cells in vitro. Toxicol Appl Pharmacol. 1996. 141:195–204.9. Berra A, Dutt JE, Nouri M, Foster CS. Heat shock protein expression in human conjunctiva. Invest Ophthalmol Vis Sci. 1994. 35:352–357.10. Polla BS, Cossarizza A. Feige U, Morimoto RI, Yahara I, Polla BS, editors. Stress proteins in inflammation. Stress-inducible cellular responses. 1996. 77. Basel: Birkhauser Verlag;375–391.
Article11. Ritossa F. A new puffing pattern induced by temperature shock and DNP in Drosophila. Experimentia. 1962. 18:571–573.
Article12. Tissieres A, Mitchell HK, Tracy UM. Protein synthesis in salivary glands of Drosophila melanogaster: Relation to chromosome puffs. J Mol Biol. 1974. 84:389–398.13. Jakob U, Buchner J. Assisting spontaneity: the role of HSP 90 and small HSP as molecular chaperones. Trends Biochem Sci. 1994. 19:205–211.14. Muchowski PJ, Valdez MM, Clark JI. AlphaB-crystallin selectively targets intermediate filament proteins during thermal stress. Invest Ophthalmol Vis Sci. 1999. 40:951–958.15. Bellmann K, Wenz A, Radons J, Burkart V, Kleemann R, Kolb H. Heat shock induces resistance in rat pancreatic islet cells against nitric oxide, oxygen radicals and streptozotocin toxicity in vitro. J Clin Invest. 1995. 95:2840–2845.
Article16. Laios E, Rebeyka IM, Prody CA. Characterization of cold-induced heat shock protein expression in neonatal rat cardiomyocytes. Mol Cell Biochem. 1997. 173:153–159.17. Kim YM, de Vera ME, Watkins SC, Billar TR. Nitric oxide protects cultured rat hepatocytes from tumor necrosis factor-α-induced apoptosis by inducing heat shock protein 70 expression. J Biol Chem. 1997. 272:1402–1411.
Article18. D'Souza CA, Rush SJ, Brown IR. Effect of hyperthermia on the transcription rate of heat-shock genes in the rabbit cerebellum and retina assayed by nuclear run-ons. J Neurosci Res. 1998. 52:538–548.19. Bellmann K, Jäättela M, Wissing D, Burkart V, Kolb H. Heat shock protein HSP 70 overexpression confers resistance against nitric oxide. FEBS Lett. 1996. 391:185–188.20. Han JA, Kwak IH, Kim JC. Ultrastructural changes of corneal edema in endotoxin-induced uveitis model. J Korean Ophthalmol Soc. 1999. 40:2103–2110.21. Joo MJ, Lee KJ, Shin KH, Kim JC. Nitric oxide-mediated neurotoxicity after excimer laser photorefractive keratectomy. J Korean Ophthalmol Soc. 1998. 40:688–698.22. Ryou JH, Bae NY, Shyn KH, Kim JC. The change of interleukin-1β and nitric oxide in tear after excimer laser photorefractive keratectomy. J Korean Ophthalmol Soc. 1998. 39:25–36.23. Jäätela M, Wissing D, Bauer PA, Li GC. Major heat shock protein HSP 70 protects tumor cells from tumor necrosis factor cytotoxicity. EMBO J. 1992. 11:3507–3512.24. Singh NP, McCoy MT, Tice RR, Schneider EL. Modification of alkaline microgel electrophoresis for sensitive detection of DNA damage. Int J Radiat Biol. 1994. 66:23–28.25. Singh PN, Michael TM, Raymond RT, Edward L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988. 175:184–191.
Article26. Kitazawa T, Tokoro T, Ishii Y. The efficacy of cooling on eximer laser photorefractive keratectomy in the rabbit eye. Surv Ophthalmol. 1997. 42:S82–S88.27. Miyamoto T, Saika S, Yamanaka A, Kawashima Y, Suzuki Y, Ohnishi Y. Wound healing in rabbit corneas after photorefractive keratectomy and laser in situ keratomileusis. J Cataract Refract Surg. 2003. 29:153–158.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Induced Heat Shock Protein 33 in Human Corneal Epithelial Cell
- The Protective Effect of Induced Heat Shock Protein in Human Corneal Epithelial Cells
- Expression of Heat Shock Protein 70 in Human Skin Cells as a Photoprotective Function after UV Exposure
- Expression of Heat Shock Protein 70 Family in Melanocytes
- Expression of Heat Shock Protein 70 m-RNA in Rat Bladder Overdistended by Diuresis