Ann Dermatol.
2008 Dec;20(4):184-189. 10.5021/ad.2008.20.4.184.
Expression of Heat Shock Protein 70 in Human Skin Cells as a Photoprotective Function after UV Exposure
- Affiliations
-
- 1Department of Dermatology, College of Medicine, Soonchunhyang University, Seoul, Korea. snolomas@hosp.sch.ac.kr
- KMID: 2156390
- DOI: http://doi.org/10.5021/ad.2008.20.4.184
Abstract
-
BACKGROUND: Human skin is exposed to various environmental stresses, such as heat, cold, and ultraviolet (UV) radiation. Heat shock proteins (HSPs) induced by temperature elevations, as a physiologic response to mediate repair mechanisms and reduce cellular damage.
OBJECTIVE
The purpose of this study was to investigate the induction of HSPs in human skin cells after UV exposure.
METHODS
We performed immunoblotting using a specific monoclonal antibody to the HSP70 family, one of the best-conserved stress proteins in humans, with cultured normal human keratinocytes, A431 cells, human melanocytes, SK30 cells, and human dermal fibroblasts (HDF).
RESULTS
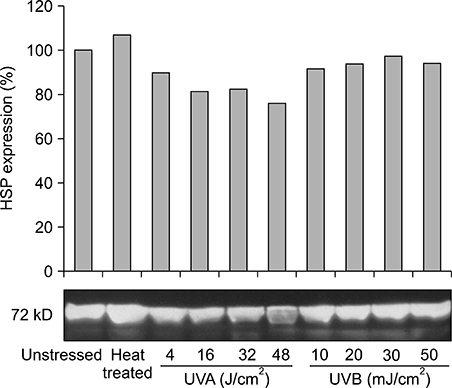
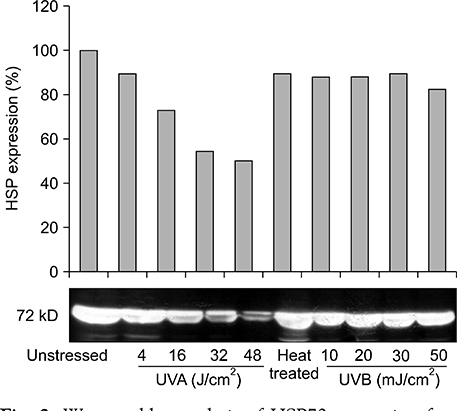
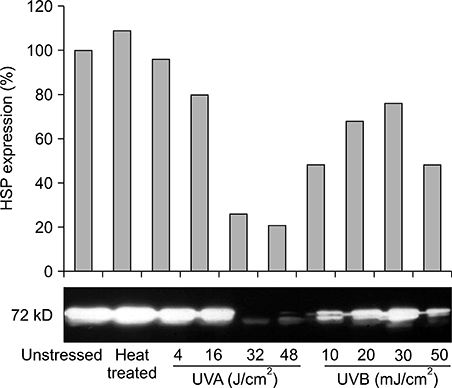
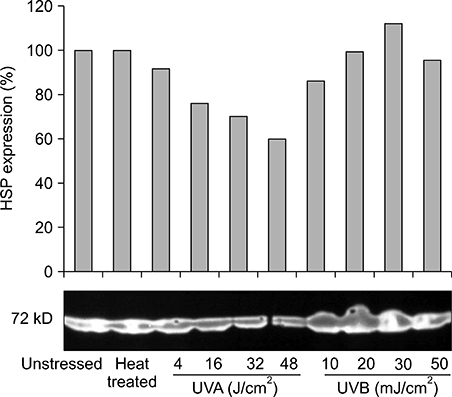
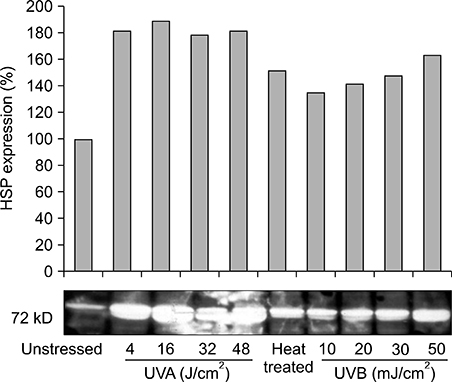
Our results indicated that high expression of HSP70 in the unstressed state was noted in epidermal cells, including normal human keratinocytes, A431 cells, human melanocytes, and SK30 cells, but epidermal cells showed no additional up-regulation of HSP70 after UV irradiation. On the other hand, HDF expressed very small amounts of HSP70 at baseline, but significantly higher amounts of HSP70 after UV exposure.
CONCLUSION
These findings suggest that constitutive expression of HSP70 in epidermal cells may be an important mechanism for protection of the human epidermis from environmental stresses, such as sunlight exposure.
Keyword
MeSH Terms
Figure
Reference
-
1. Ritossa F. A new puffing pattern induced by temperature shock and DNP in drosophila. Experientia. 1962; 18:571–573.
Article2. Ritossa F. Discovery of the heat shock response. Cell Stress Chaperones. 1996; 1:97–98.
Article3. Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988; 22:631–677.
Article4. Jonak C, Klosner G, Trautinger F. Heat shock proteins in the skin. Int J Cosmet Sci. 2006; 28:233–241.
Article5. Maytin EV. Differential effects of heat shock and UVB light upon stress protein expression in epidermal keratinocytes. J Biol Chem. 1992; 267:23189–23196.
Article6. Holland DB, Roberts SG, Wood EJ, Cunliffe WJ. Cold shock induces the synthesis of stress proteins in human keratinocytes. J Invest Dermatol. 1993; 101:196–199.
Article7. Muramatsu T, Yamashina Y, Tada H, Kobayashi N, Yamaji M, Ohno H, et al. 8-methoxypsoralen plus UVA induces the 72 kDa heat shock protein in organ-cultured normal human skin. Photochem Photobiol. 1993; 58:809–812.
Article8. Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992; 72:1063–1081.
Article9. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193:265–275.
Article10. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970; 227:680–685.
Article11. Hendrick JP, Hartl FU. Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem. 1993; 62:349–384.
Article12. Craig EA, Weissman JS, Horwich AL. Heat shock proteins and molecular chaperones: mediators of protein conformation and turnover in the cell. Cell. 1994; 78:365–372.
Article13. Wynn RM, Davie JR, Cox RP, Chuang DT. Molecular chaperones: heat-shock proteins, foldases, and matchmakers. J Lab Clin Med. 1994; 124:31–36.14. Minowada G, Welch WJ. Clinical implications of the stress response. J Clin Invest. 1995; 95:3–12.
Article15. Welch WJ, Suhan JP. Cellular and biochemical events in mammalian cells during and after recovery from physiological stress. J Cell Biol. 1986; 103:2035–2052.
Article16. Maytin EV. Heat shock proteins and molecular chaperones: implications for adaptive responses in the skin. J Invest Dermatol. 1995; 104:448–455.
Article17. Pelham HR. Hsp70 accelerates the recovery of nucleolar morphology after heat shock. EMBO J. 1984; 3:3095–3100..
Article18. Ungewickell E. The 70-kd mammalian heat shock proteins are structurally and functionally related to the uncoating protein that releases clathrin triskelia from coated vesicles. EMBO J. 1985; 4:3385–3391.
Article19. Beckmann RP, Mizzen LE, Welch WJ. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990; 248:850–854.
Article20. Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992; 355:33–45.
Article21. Trent JD, Nimmesgern E, Wall JS, Hartl FU, Horwich AL. A molecular chaperone from a thermophilic archaebacterium is related to the eukaryotic protein t-complex polypeptide-1. Nature. 1991; 354:490–493.
Article22. Horwich AL, Willison KR. Protein folding in the cell: functions of two families of molecular chaperone, hsp 60 and TF55-TCP1. Philos Trans R Soc Lond B Biol Sci. 1993; 339:313–325.
Article23. Frydman J, Nimmesgern E, Ohtsuka K, Hartl FU. Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones. . Nature. 1994; 370:111–117.
Article24. Trautinger F, Trautinger I, Kindas-Mugge I, Metze D, Luger TA. Human keratinocytes in vivo and in vitro constitutively express the 72-kD heat shock protein. J Invest Dermatol. 1993; 101:334–338.
Article25. Charveron M, Calvo M, Gall Y. Cell stress and implications of the heat-shock response in skin. Cell Biol Toxicol. 1995; 11:161–165.
Article26. Laplante AF, Moulin V, Auger FA, Landry J, Li H, Morrow G, et al. Expression of heat shock proteins in mouse skin during wound healing. J Histochem Cytochem. 1998; 46:1291–1301.
Article27. Muramatsu T, Tada H, Kobayashi N, Yamji M, Shirai T, Ohnishi T. Induction of the 72-kD heat shock protein in organ-cultured normal human skin. J Invest Dermatol. 1992; 98:786–790.
Article28. Brunet S, Giacomoni PU. Heat shock mRNA in mouse epidermis after UV irradiation. Mutat Res. 1989; 219:217–224.
Article29. Simon MM, Reikerstorfer A, Schwarz A, Krone C, Luger TA, Jaattela M, et al. Heat shock protein 70 overexpression affects the response to ultraviolet light in murine fibroblasts. Evidence for increased cell viability and suppression of cytokine release. J Clin Invest. 1995; 95:926–933.
Article30. Keyse SM, Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci USA. 1989; 86:99–103.
Article31. Trautinger F, Kokesch C, Klosner G, Knobler RM, Kindas-Mugge I. Expression of the 72-kD heat shock protein is induced by ultraviolet A radiation in a human fibrosarcoma cell line. Exp Dermatol. 1999; 8:187–192.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Protective Effect of Induced Heat Shock Protein in Human Corneal Epithelial Cells
- Expression of Heat Shock Protein 70 Family in Melanocytes
- Expression of Heat Shock Protein 70 m-RNA in Rat Bladder Overdistended by Diuresis
- Environmental factors regulating the expression of Porphyromonas gingivalis heat shock protein
- Expression of Heat Shock Protein 70 in Vein Endothelial Cells Induced by Shear Stress