Immune Netw.
2014 Dec;14(6):307-320. 10.4110/in.2014.14.6.307.
Characterization of Proinflammatory Responses and Innate Signaling Activation in Macrophages Infected with Mycobacterium scrofulaceum
- Affiliations
-
- 1Center of Inflammation, Infection & Immunity, Institute for Biomedical Sciences, Georgia State University, Atlanta, GA, USA.
- 2Department of Infection Biology, Chungnam National University School of Medicine, Daejeon 301-747, Korea. yjaemin0@cnu.ac.kr
- 3Department of Microbiology, Chungnam National University School of Medicine, Daejeon 301-747, Korea.
- 4Infection Signaling Network Research Center, Chungnam National University School of Medicine, Daejeon 301-747, Korea.
- 5Department of Pathology, Chungnam National University School of Medicine, Daejeon 301-747, Korea.
- KMID: 2150821
- DOI: http://doi.org/10.4110/in.2014.14.6.307
Abstract
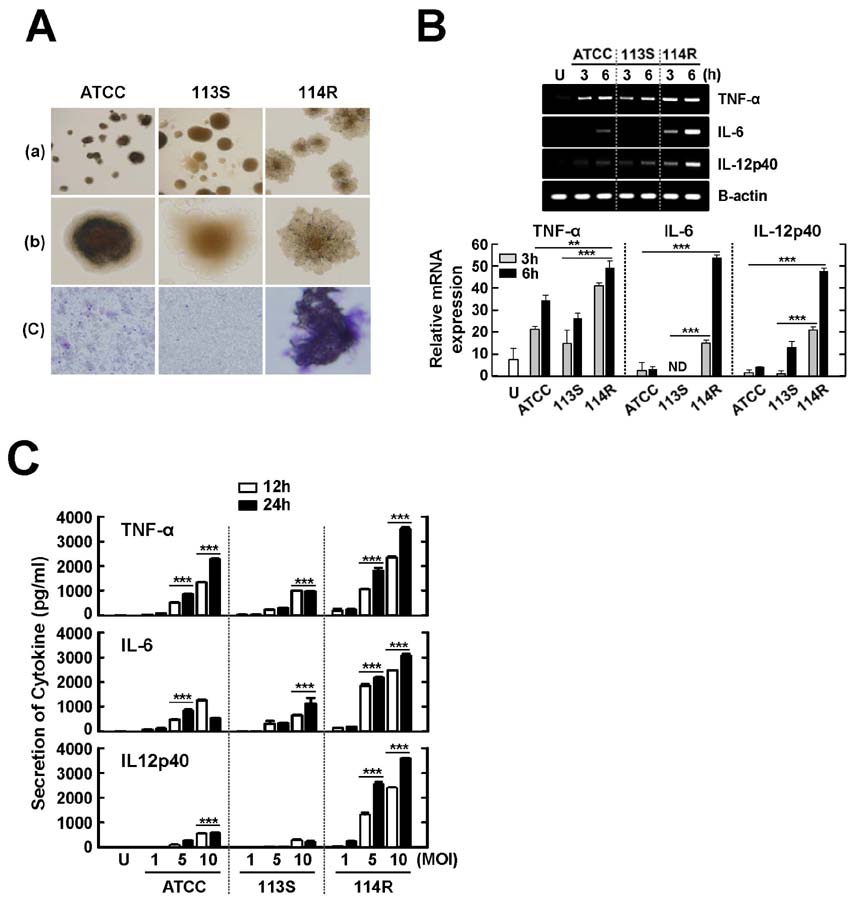
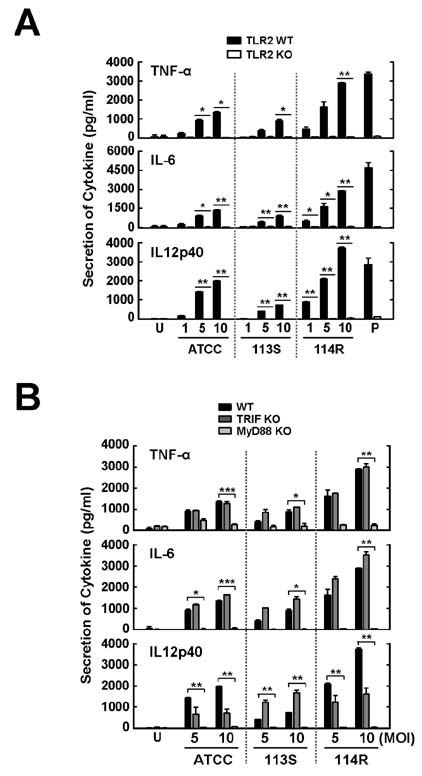
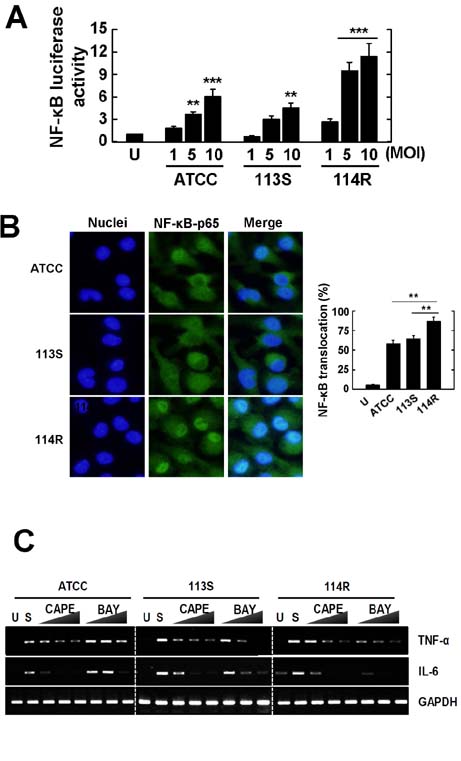
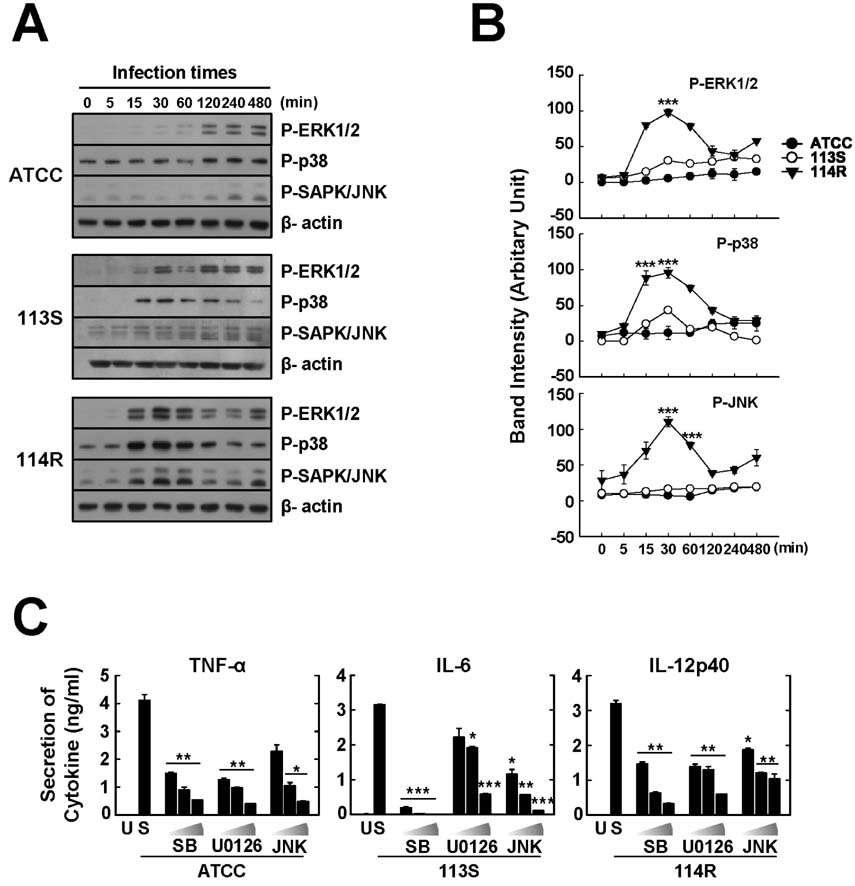
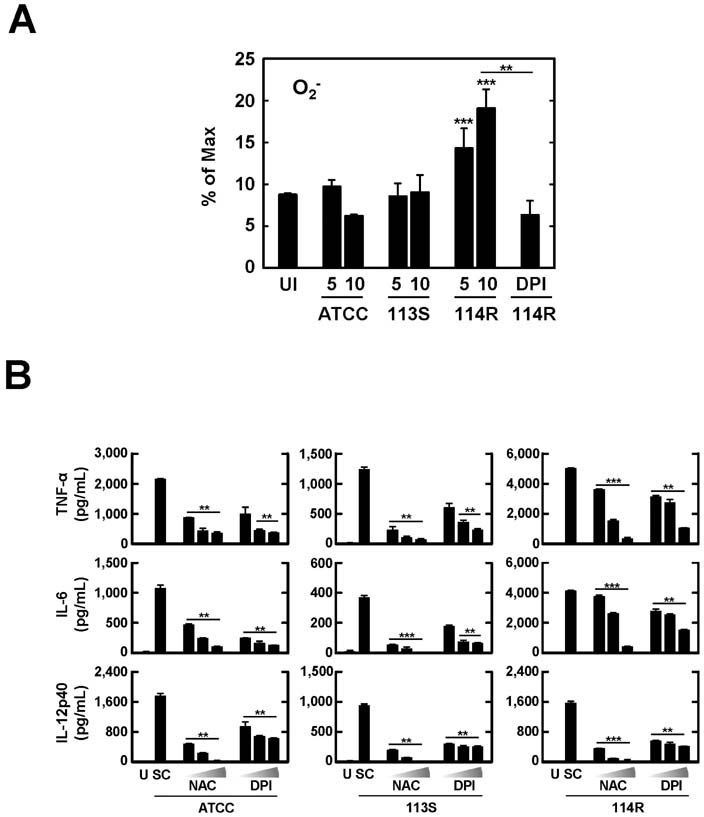
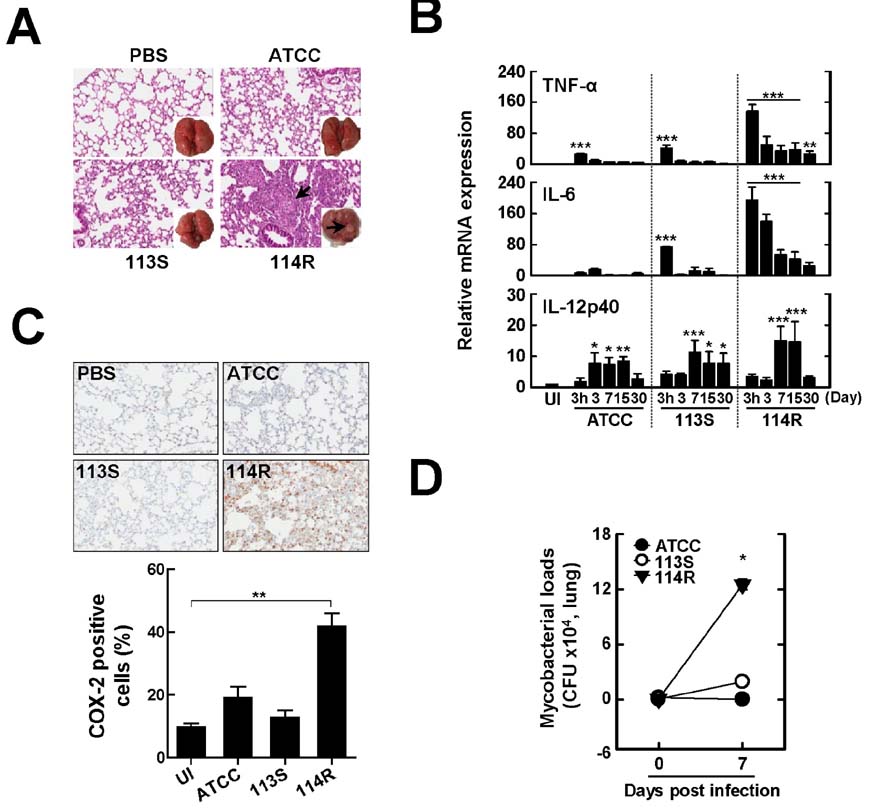
- Mycobacterium scrofulaceum is an environmental and slow-growing atypical mycobacterium. Emerging evidence suggests that M. scrofulaceum infection is associated with cervical lymphadenitis in children and pulmonary or systemic infections in immunocompromised adults. However, the nature of host innate immune responses to M. scrofulaceum remains unclear. In this study, we examined the innate immune responses in murine bone marrow-derived macrophages (BMDMs) infected with different M. scrofulaceum strains including ATCC type strains and two clinically isolated strains (rough and smooth types). All three strains resulted in the production of proinflammatory cytokines in BMDMs mediated through toll-like receptor-2 and the adaptor MyD88. Activation of MAPKs (extracellular signal-regulated kinase 1/2, and p38, and c-Jun N-terminal kinase) and nuclear receptor (NF)-kappaB together with intracellular reactive oxygen species generation were required for the expression of proinflammatory cytokines in BMDMs. In addition, the rough morphotypes of M. scrofulaceum clinical strains induced higher levels of proinflammatory cytokines, MAPK and NF-kappaB activation, and ROS production than other strains. When mice were infected with different M. scrofulaceum strains, those infected with the rough strain showed the greatest hepatosplenomegaly, granulomatous lesions, and immune cell infiltration in the lungs. Notably, the bacterial load was higher in mice infected with rough colonies than in mice infected with ATCC or smooth strains. Collectively, these data indicate that rough M. scrofulaceum induces higher inflammatory responses and virulence than ATCC or smooth strains.
Keyword
MeSH Terms
Figure
Reference
-
1. Sanders JW, Walsh AD, Snider RL, Sahn EE. Disseminated Mycobacterium scrofulaceum infection: a potentially treatable complication of AIDS. Clin Infect Dis. 1995; 20:549.
Article2. Hsueh PR, Hsiue TR, Jarn JJ, Ho SW, Hsieh WC. Disseminated infection due to Mycobacterium scrofulaceum in an immunocompetent host. Clin Infect Dis. 1996; 22:159–161.
Article3. Ryoo SW, Shin S, Shim MS, Park YS, Lew WJ, Park SN, Park YK, Kang S. Spread of nontuberculous mycobacteria from 1993 to 2006 in Koreans. J Clin Lab Anal. 2008; 22:415–420.
Article4. Falkinham JO III. Factors influencing the chlorine susceptibility of Mycobacterium avium, Mycobacterium intracellulare, and Mycobacterium scrofulaceum. Appl Environ Microbiol. 2003; 69:5685–5689.
Article5. Rosenzweig DY. "Atypical" mycobacterioses. Clin Chest Med. 1980; 1:273–284.
Article6. Verrall AJ, Netea MG, Alisjahbana B, Hill PC, van CR. Early clearance of Mycobacterium tuberculosis: a new frontier in prevention. Immunology. 2014; 141:506–513.
Article7. Jo EK, Yang CS, Choi CH, Harding CV. Intracellular signalling cascades regulating innate immune responses to Mycobacteria: branching out from Toll-like receptors. Cell Microbiol. 2007; 9:1087–1098.
Article8. Shin DM, Yang CS, Yuk JM, Lee JY, Kim KH, Shin SJ, Takahara K, Lee SJ, Jo EK. Mycobacterium abscessus activates the macrophage innate immune response via a physical and functional interaction between TLR2 and dectin-1. Cell Microbiol. 2008; 10:1608–1621.
Article9. Lee HM, Shin DM, Choi DK, Lee ZW, Kim KH, Yuk JM, Kim CD, Lee JH, Jo EK. Innate immune responses to Mycobacterium ulcerans via toll-like receptors and dectin-1 in human keratinocytes. Cell Microbiol. 2009; 11:678–692.
Article10. Torrado E, Cooper AM. Cytokines in the balance of protection and pathology during mycobacterial infections. Adv Exp Med Biol. 2013; 783:121–140.
Article11. Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006; 13:816–825.
Article12. Basu J, Shin DM, Jo EK. Mycobacterial signaling through toll-like receptors. Front Cell Infect Microbiol. 2012; 2:145.
Article13. Quigley M, Martinez J, Huang X, Yang Y. A critical role for direct TLR2-MyD88 signaling in CD8 T-cell clonal expansion and memory formation following vaccinia viral infection. Blood. 2009; 113:2256–2264.
Article14. Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014; 24:R453–R462.
Article15. Yang CS, Shin DM, Kim KH, Lee ZW, Lee CH, Park SG, Bae YS, Jo EK. NADPH oxidase 2 interaction with TLR2 is required for efficient innate immune responses to mycobacteria via cathelicidin expression. J Immunol. 2009; 182:3696–3705.
Article16. Yuk JM, Shin DM, Yang CS, Kim KH, An SJ, Rho J, Park JK, Jo EK. Role of apoptosis-regulating signal kinase 1 in innate immune responses by Mycobacterium bovis bacillus Calmette-Guerin. Immunol Cell Biol. 2009; 87:100–107.
Article17. Shin DM, Yuk JM, Lee HM, Lee SH, Son JW, Harding CV, Kim JM, Modlin RL, Jo EK. Mycobacterial lipoprotein activates autophagy via TLR2/1/CD14 and a functional vitamin D receptor signalling. Cell Microbiol. 2010; 12:1648–1665.
Article18. Howard ST, Rhoades E, Recht J, Pang X, Alsup A, Kolter R, Lyons CR, Byrd TF. Spontaneous reversion of Mycobacterium abscessus from a smooth to a rough morphotype is associated with reduced expression of glycopeptidolipid and reacquisition of an invasive phenotype. Microbiology. 2006; 152:1581–1590.
Article19. Byrd TF, Lyons CR. Preliminary characterization of a Mycobacterium abscessus mutant in human and murine models of infection. Infect Immun. 1999; 67:4700–4707.
Article20. Kim TS, Kim YS, Yoo H, Park YK, Jo EK. Mycobacterium massiliense induces inflammatory responses in macrophages through Toll-like receptor 2 and c-Jun N-terminal kinase. J Clin Immunol. 2014; 34:212–223.
Article21. Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011; 34:637–650.
Article22. Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009; 1:a000034.23. Cruz-Knight W, Blake-Gumbs L. Tuberculosis: an overview. Prim Care. 2013; 40:743–756.24. Huynh KK, Joshi SA, Brown EJ. A delicate dance: host response to mycobacteria. Curr Opin Immunol. 2011; 23:464–472.
Article25. Marazzi MG, Chapgier A, Defilippi AC, Pistoia V, Mangini S, Savioli C, Dell'Acqua A, Feinberg J, Tortoli E, Casanova JL. Disseminated Mycobacterium scrofulaceum infection in a child with interferon-gamma receptor 1 deficiency. Int J Infect Dis. 2010; 14:e167–e170.26. Haas WH, Kirschner P, Ziesing S, Bremer HJ, Bottger EC. Cervical lymphadenitis in a child caused by a previously unknown mycobacterium. J Infect Dis. 1993; 167:237–240.
Article27. Tortoli E, Kirschner P, Springer B, Bartoloni A, Burrini C, Mantella A, Scagnelli M, Scarparo C, Simonetti MT, Bottger EC. . Cervical lymphadenitis due to an unusual mycobacterium. Eur J Clin Microbiol Infect Dis. 1997; 16:308–311.28. Kamala T, Paramasivan CN, Herbert D, Venkatesan P, Prabhakar R. Isolation and Identification of Environmental Mycobacteria in the Mycobacterium bovis BCG Trial Area of South India. Appl Environ Microbiol. 1994; 60:2180–2183.
Article29. Torvinen E, Suomalainen S, Paulin L, Kusnetsov J. Mycobacteria in Finnish cooling tower waters. APMIS. 2014; 122:353–358.
Article30. Puthanakit T, Oberdorfer P, Ukarapol N, Akarathum N, Punjaisee S, Sirisanthana T, Sirisanthana V. Immune reconstitution syndrome from nontuberculous mycobacterial infection after initiation of antiretroviral therapy in children with HIV infection. Pediatr Infect Dis J. 2006; 25:645–648.
Article31. Brandt L, Feino CJ, Weinreich OA, Chilima B, Hirsch P, Appelberg R, Andersen P. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect Immun. 2002; 70:672–678.
Article32. Flaherty DK, Vesosky B, Beamer GL, Stromberg P, Turner J. Exposure to Mycobacterium avium can modulate established immunity against Mycobacterium tuberculosis infection generated by Mycobacterium bovis BCG vaccination. J Leukoc Biol. 2006; 80:1262–1271.
Article33. Barrow WW, Brennan PJ. Isolation in high frequency of rough variants of Mycobacterium intracellulare lacking C-mycoside glycopeptidolipid antigens. J Bacteriol. 1982; 150:381–384.
Article34. Recht J, Kolter R. Glycopeptidolipid acetylation affects sliding motility and biofilm formation in Mycobacterium smegmatis. J Bacteriol. 2001; 183:5718–5724.
Article35. Bernut A, Herrmann JL, Kissa K, Dubremetz JF, Gaillard JL, Lutfalla G, Kremer L. Mycobacterium abscessus cording prevents phagocytosis and promotes abscess formation. Proc Natl Acad Sci U S A. 2014; 111:E943–E952.36. Rhoades ER, Archambault AS, Greendyke R, Hsu FF, Streeter C, Byrd TF. Mycobacterium abscessus Glycopeptidolipids mask underlying cell wall phosphatidyl-myo-inositol mannosides blocking induction of human macrophage TNF-alpha by preventing interaction with TLR2. J Immunol. 2009; 183:1997–2007.
Article37. Jonsson B, Ridell M, Wold AE. Phagocytosis and cytokine response to rough and smooth colony variants of Mycobacterium abscessus by human peripheral blood mononuclear cells. APMIS. 2013; 121:45–55.
Article38. Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999; 163:3920–3927.39. Wang T, Lafuse WP, Zwilling BS. Regulation of toll-like receptor 2 expression by macrophages following Mycobacterium avium infection. J Immunol. 2000; 165:6308–6313.
Article40. Vignal C, Guerardel Y, Kremer L, Masson M, Legrand D, Mazurier J, Elass E. Lipomannans, but not lipoarabinomannans, purified from Mycobacterium chelonae and Mycobacterium kansasii induce TNF-alpha and IL-8 secretion by a CD14-toll-like receptor 2-dependent mechanism. J Immunol. 2003; 171:2014–2023.
Article41. Kleinnijenhuis J, Oosting M, Joosten LA, Netea MG, Van CR. Innate immune recognition of Mycobacterium tuberculosis. Clin Dev Immunol. 2011; 2011:405310.42. Fremond CM, Togbe D, Doz E, Rose S, Vasseur V, Maillet I, Jacobs M, Ryffel B, Quesniaux VF. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J Immunol. 2007; 179:1178–1189.
Article43. Yoshida A, Inagawa H, Kohchi C, Nishizawa T, Soma G. The role of toll-like receptor 2 in survival strategies of Mycobacterium tuberculosis in macrophage phagosomes. Anticancer Res. 2009; 29:907–910.44. Koul A, Herget T, Klebl B, Ullrich A. Interplay between mycobacteria and host signalling pathways. Nat Rev Microbiol. 2004; 2:189–202.
Article45. Schorey JS, Cooper AM. Macrophage signalling upon mycobacterial infection: the MAP kinases lead the way. Cell Microbiol. 2003; 5:133–142.
Article46. Jung SB, Yang CS, Lee JS, Shin AR, Jung SS, Son JW, Harding CV, Kim HJ, Park JK, Paik TH, Song CH, Jo EK. The mycobacterial 38-kilodalton glycolipoprotein antigen activates the mitogen-activated protein kinase pathway and release of proinflammatory cytokines through Toll-like receptors 2 and 4 in human monocytes. Infect Immun. 2006; 74:2686–2696.
Article47. Sim YS, Kim SY, Kim EJ, Shin SJ, Koh WJ. Impaired Expression of MAPK Is Associated with the Downregulation of TNF-alpha, IL-6, and IL-10 in Mycobacterium abscessus Lung Disease. Tuberc Respir Dis (Seoul). 2012; 72:275–283.
Article48. Launois P, Niang M, Dieye A, Sarthou JL, Rivier F, Millan J. Human phagocyte respiratory burst by Mycobacterium bovis BCG and M. leprae: functional activation by BCG is mediated by complement and its receptors on monocytes. Int J Lepr Other Mycobact Dis. 1992; 60:225–233.49. Ferrari CK, Souto PC, Franca EL, Honorio-Franca AC. Oxidative and nitrosative stress on phagocytes function: from effective defense to immunity evasion mechanisms. Arch Immunol Ther Exp (Warsz). 2011; 59:441–448.
Article50. Vazquez-Torres A, Fang FC. Oxygen-dependent anti-Salmonella activity of macrophages. Trends Microbiol. 2001; 9:29–33.
Article51. Lim YJ, Choi HH, Choi JA, Jeong JA, Cho SN, Lee JH, Park JB, Kim HJ, Song CH. Mycobacterium kansasii-induced death of murine macrophages involves endoplasmic reticulum stress responses mediated by reactive oxygen species generation or calpain activation. Apoptosis. 2013; 18:150–159.
Article52. Kim JJ, Lee HM, Shin DM, Kim W, Yuk JM, Jin HS, Lee SH, Cha GH, Kim JM, Lee ZW, Shin SJ, Yoo H, Park YK, Park JB, Chung J, Yoshimori T, Jo EK. Host cell autophagy activated by antibiotics is required for their effective antimycobacterial drug action. Cell Host Microbe. 2012; 11:457–468.
Article53. Vilcheze C, Hartman T, Weinrick B, Jacobs WR Jr. Mycobacterium tuberculosis is extraordinarily sensitive to killing by a vitamin C-induced Fenton reaction. Nat Commun. 2013; 4:1881.54. Romero MM, Balboa L, Basile JI, Lopez B, Ritacco V, de la Barrera SS, Sasiain MC, Barrera L, Aleman M. Clinical isolates of Mycobacterium tuberculosis differ in their ability to induce respiratory burst and apoptosis in neutrophils as a possible mechanism of immune escape. Clin Dev Immunol. 2012; 2012:152546.55. Reddy VM, Luna-Herrera J, Gangadharam PR. Pathobiological significance of colony morphology in Mycobacterium avium complex. Microb Pathog. 1996; 21:97–109.
Article56. Sohn H, Kim HJ, Kim JM, Jung KO, Koh WJ, Shin SJ. High virulent clinical isolates of Mycobacterium abscessus from patients with the upper lobe fibrocavitary form of pulmonary disease. Microb Pathog. 2009; 47:321–328.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Toll-like Receptors and Innate Immunity
- Effector Pathways of Toll-like Receptor-inducible Innate Immune Responses in Macrophages
- Mycobacterial Heparin-binding Hemagglutinin Antigen Activates Inflammatory Responses through PI3-K/Akt, NF-kappaB, and MAPK Pathways
- Neonatal innate immunity and Toll-like receptor
- Identification and Functional Characterization of Differentially Expressed Genes in Human-derived Monocytic Cell Line U937 Infected with Mycobacterium tuberculosis H37Rv and Mycobacterium marinum: Comparative Evaluation of IL-8