Immune Netw.
2009 Jun;9(3):90-97. 10.4110/in.2009.9.3.90.
The Stimulation of CD147 Induces MMP-9 Expression through ERK and NF-kappaB in Macrophages: Implication for Atherosclerosis
- Affiliations
-
- 1The School of Life Sciences and Biotechnology, Kyungpook National University, Daegu 702-701, Korea. whl@knu.ac.kr
- 2Department of Pharmacology, School of Medicine, Kyungpook National University, Daegu 702-701, Korea.
- KMID: 2150647
- DOI: http://doi.org/10.4110/in.2009.9.3.90
Abstract
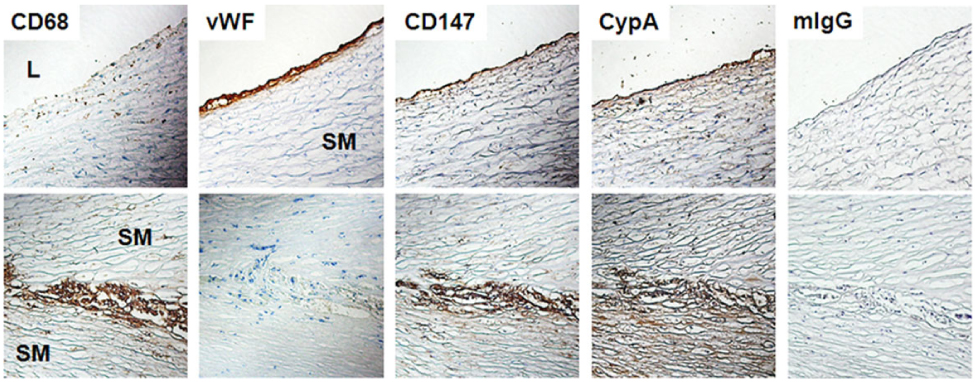
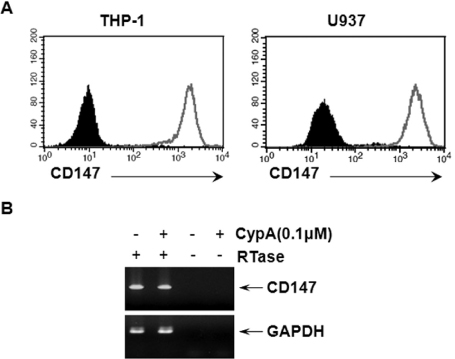
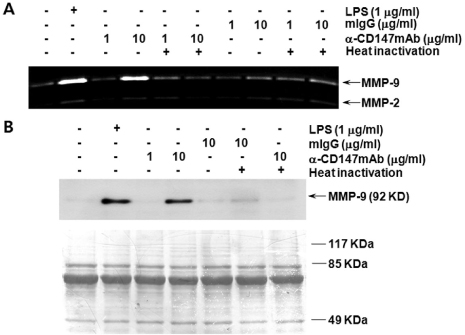
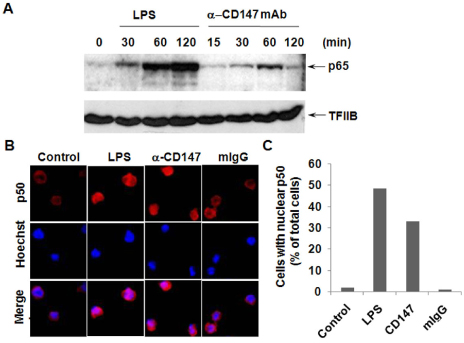
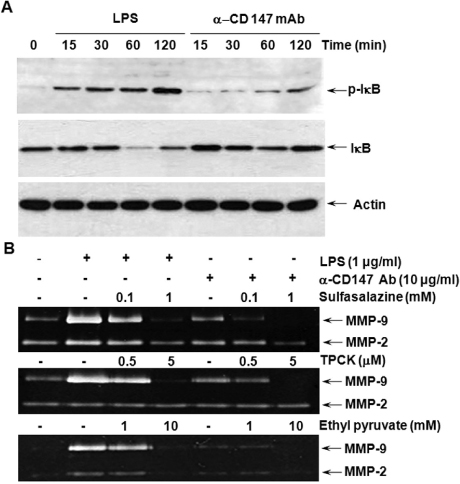
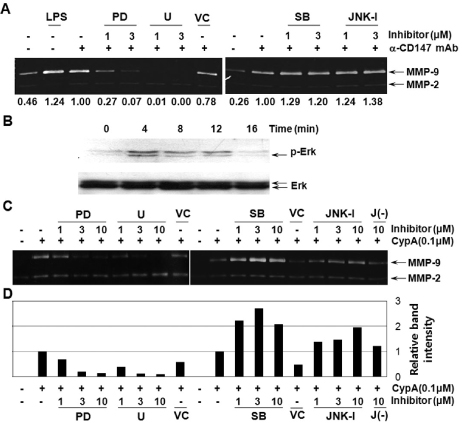
- BACKGROUND: CD147, as a cellular receptor for cyclophilin A (CypA), is a multifunctional protein involved in tumor invasion, inflammation, tissue remodeling, neural function, and reproduction. Recent observations showing the expression of CD147 in leukocytes indicate that this molecule may have roles in inflammation. METHODS: In order to investigate the role of CD147 and its ligand in the pathogenesis of atherosclerosis, human atherosclerotic plaques were analyzed for the expression pattern of CD147 and CypA. The cellular responses and signaling molecules activated by the stimulation of CD147 were then investigated in the human macrophage cell line, THP-1, which expresses high basal level of CD147 on the cell surface. RESULTS: Staining of both CD147 and CypA was detected in endothelial cell layers facing the lumen and macrophage-rich areas. Stimulation of CD147 with its specific monoclonal antibody induced the expression of matrix metalloproteinase (MMP)-9 in THP-1 cells and it was suppressed by inhibitors of both ERK and NF-kappaB. Accordingly, the stimulation of CD147 was observed to induce phosphorylation of ERK, phosphorylation-associated degradation of IkappaB, and nuclear translocation of NF-kappaB p65 and p50 subunits. CONCLUSION: These results suggest that CD147 mediates the inflammatory activation of macrophages that leads to the induction of MMP-9 expression, which could play a role in the pathogenesis of inflammatory diseases such as atherosclerosis.
Keyword
MeSH Terms
Figure
Reference
-
1. Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H, Nabeshima K. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995. 55:434–439.2. Biswas C. Tumor cell stimulation of collagenase production by fibroblasts. Biochem Biophys Res Commun. 1982. 109:1026–1034.
Article3. Ellis SM, Nabeshima K, Biswas C. Monoclonal antibody preparation and purification of a tumor cell collagenase-stimulatory factor. Cancer Res. 1989. 49:3385–3391.4. Kasinrerk W, Fiebiger E, Stefanova I, Baumruker T, Knapp W, Stockinger H. Human leukocyte activation antigen M6, a member of the Ig superfamily, is the species homologue of rat OX-47, mouse basigin, and chicken HT7 molecule. J Immunol. 1992. 149:847–854.5. Kataoka H, DeCastro R, Zucker S, Biswas C. Tumor cell-derived collagenase-stimulatory factor increases expression of interstitial collagenase, stromelysin, and 72-kDa gelatinase. Cancer Res. 1993. 53:3154–3158.6. Nabeshima K, Lane WS, Biswas C. Partial sequencing and characterization of the tumor cell-derived collagenase stimulatory factor. Arch Biochem Biophys. 1991. 285:90–96.
Article7. Caudroy S, Polette M, Nawrocki-Raby B, Cao J, Toole BP, Zucker S, Birembaut P. EMMPRIN-mediated MMP regulation in tumor and endothelial cells. Clin Exp Metastasis. 2002. 19:697–702.8. Tang Y, Nakada MT, Kesavan P, McCabe F, Millar H, Rafferty P, Bugelski P, Yan L. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res. 2005. 65:3193–3199.
Article9. Foda HD, Rollo EE, Drews M, Conner C, Appelt K, Shalinsky DR, Zucker S. Ventilator-induced lung injury up-regulates and activates gelatinases and EMMPRIN: attenuation by the synthetic matrix metalloproteinase inhibitor, Prinomastat (AG3340). Am J Respir Cell Mol Biol. 2001. 25:717–724.
Article10. Konttinen YT, Li TF, Mandelin J, Liljestrom M, Sorsa T, Santavirta S, Virtanen I. Increased expression of extracellular matrix metalloproteinase inducer in rheumatoid synovium. Arthritis Rheum. 2000. 43:275–280.
Article11. Major TC, Liang L, Lu X, Rosebury W, Bocan TM. Extracellular matrix metalloproteinase inducer (EMMPRIN) is induced upon monocyte differentiation and is expressed in human atheroma. Arterioscler Thromb Vasc Biol. 2002. 22:1200–1207.
Article12. Shackel NA, McGuinness PH, Abbott CA, Gorrell MD, McCaughan GW. Insights into the pathobiology of hepatitis C virus-associated cirrhosis: analysis of intrahepatic differential gene expression. Am J Pathol. 2002. 160:641–654.
Article13. Spinale FG, Coker ML, Heung LJ, Bond BR, Gunasinghe HR, Etoh T, Goldberg AT, Zellner JL, Crumbley AJ. A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation. 2000. 102:1944–1949.
Article14. Pushkarsky T, Zybarth G, Dubrovsky L, Yurchenko V, Tang H, Guo H, Toole B, Sherry B, Bukrinsky M. CD147 facilitates HIV-1 infection by interacting with virus-associated cyclophilin A. Proc Natl Acad Sci U S A. 2001. 98:6360–6365.
Article15. Yurchenko V, O'Connor M, Dai WW, Guo H, Toole B, Sherry B, Bukrinsky M. CD147 is a signaling receptor for cyclophilin B. Biochem Biophys Res Commun. 2001. 288:786–788.
Article16. Sherry B, Yarlett N, Strupp A, Cerami A. Identification of cyclophilin as a proinflammatory secretory product of lipopolysaccharide-activated macrophages. Proc Natl Acad Sci U S A. 1992. 89:3511–3515.
Article17. Jin ZG, Lungu AO, Xie L, Wang M, Wong C, Berk BC. Cyclophilin A is a proinflammatory cytokine that activates endothelial cells. Arterioscler Thromb Vasc Biol. 2004. 24:1186–1191.
Article18. Jin ZG, Melaragno MG, Liao DF, Yan C, Haendeler J, Suh YA, Lambeth JD, Berk BC. Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ Res. 2000. 87:789–796.
Article19. Kim SH, Lessner SM, Sakurai Y, Galis ZS. Cyclophilin A as a novel biphasic mediator of endothelial activation and dysfunction. Am J Pathol. 2004. 164:1567–1574.
Article20. Kim H, Kim WJ, Jeon ST, Koh EM, Cha HS, Ahn KS, Lee WH. Cyclophilin A may contribute to the inflammatory processes in rheumatoid arthritis through induction of matrix degrading enzymes and inflammatory cytokines from macrophages. Clin Immunol. 2005. 116:217–224.
Article21. Yang Y, Lu N, Zhou J, Chen ZN, Zhu P. Cyclophilin A up-regulates MMP-9 expression and adhesion of monocytes/macrophages via CD147 signalling pathway in rheumatoid arthritis. Rheumatology (Oxford). 2008. 47:1299–1310.
Article22. Damsker JM, Okwumabua I, Pushkarsky T, Arora K, Bukrinsky MI, Constant SL. Targeting the chemotactic function of CD147 reduces collagen-induced arthritis. Immunology. 2009. 126:55–62.
Article23. Damsker JM, Bukrinsky MI, Constant SL. Preferential chemotaxis of activated human CD4+ T cells by extracellular cyclophilin A. J Leukoc Biol. 2007. 82:613–618.
Article24. Schmidt R, Bultmann A, Ungerer M, Joghetaei N, Bulbul O, Thieme S, Chavakis T, Toole BP, Gawaz M, Schomig A, May AE. Extracellular matrix metalloproteinase inducer regulates matrix metalloproteinase activity in cardiovascular cells: implications in acute myocardial infarction. Circulation. 2006. 113:834–841.
Article25. Bonny C, Oberson A, Negri S, Sauser C, Schorderet DF. Cell-permeable peptide inhibitors of JNK: novel blockers of beta-cell death. Diabetes. 2001. 50:77–82.26. Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. 1980. 26:171–176.
Article27. Lee WH, Kim SH, Lee Y, Lee BB, Kwon B, Song H, Kwon BS, Park JE. Tumor necrosis factor receptor superfamily 14 is involved in atherogenesis by inducing proinflammatory cytokines and matrix metalloproteinases. Arterioscler Thromb Vasc Biol. 2001. 21:2004–2010.
Article28. Kim SH, Kang YJ, Kim WJ, Woo DK, Lee Y, Kim DI, Park YB, Kwon BS, Park JE, Lee WH. TWEAK can induce pro-inflammatory cytokines and matrix metalloproteinase-9 in macrophages. Circ J. 2004. 68:396–399.
Article29. Ruggeri ZM. Structure and function of von Willebrand factor. Thromb Haemost. 1999. 82:576–584.
Article30. Whincup PH, Danesh J, Walker M, Lennon L, Thomson A, Appleby P, Rumley A, Lowe GD. von Willebrand factor and coronary heart disease: prospective study and meta-analysis. Eur Heart J. 2002. 23:1764–1770.
Article31. Kamei M, Yoneda K, Kume N, Suzuki M, Itabe H, Matsuda K, Shimaoka T, Minami M, Yonehara S, Kita T, Kinoshita S. Scavenger receptors for oxidized lipoprotein in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007. 48:1801–1807.
Article32. Pilarczyk K, Sattler KJ, Galili O, Versari D, Olson ML, Meyer FB, Zhu XY, Lerman LO, Lerman A. Placenta growth factor expression in human atherosclerotic carotid plaques is related to plaque destabilization. Atherosclerosis. 2008. 196:333–340.
Article33. Boulos S, Meloni BP, Arthur PG, Majda B, Bojarski C, Knuckey NW. Evidence that intracellular cyclophilin A and cyclophilin A/CD147 receptor-mediated ERK1/2 signalling can protect neurons against in vitro oxidative and ischemic injury. Neurobiol Dis. 2007. 25:54–64.
Article34. Schmidt R, Bultmann A, Fischel S, Gillitzer A, Cullen P, Walch A, Jost P, Ungerer M, Tolley ND, Lindemann S, Gawaz M, Schomig A, May AE. Extracellular matrix metalloproteinase inducer (CD147) is a novel receptor on platelets, activates platelets, and augments nuclear factor kappaB-dependent inflammation in monocytes. Circ Res. 2008. 102:302–309.
Article35. Yang H, Chen J, Yang J, Qiao S, Zhao S, Yu L. Cyclophilin A is upregulated in small cell lung cancer and activates ERK1/2 signal. Biochem Biophys Res Commun. 2007. 361:763–767.
Article36. Heidinger M, Kolb H, Krell HW, Jochum M, Ries C. Modulation of autocrine TNF-alpha-stimulated matrix metalloproteinase 9 (MMP-9) expression by mitogen-activated protein kinases in THP-1 monocytic cells. Biol Chem. 2006. 387:69–78.37. Bae EM, Kim WJ, Suk K, Kang YM, Park JE, Kim WY, Choi EM, Choi BK, Kwon BS, Lee WH. Reverse signaling initiated from GITRL induces NF-kappaB activation through ERK in the inflammatory activation of macrophages. Mol Immunol. 2008. 45:523–533.
Article38. Yang H, Li M, Chai H, Yan S, Lin P, Lumsden AB, Yao Q, Chen C. Effects of cyclophilin A on cell proliferation and gene expressions in human vascular smooth muscle cells and endothelial cells. J Surg Res. 2005. 123:312–319.
Article39. Sameshima T, Nabeshima K, Toole BP, Yokogami K, Okada Y, Goya T, Koono M, Wakisaka S. Expression of emmprin (CD147), a cell surface inducer of matrix metalloproteinases, in normal human brain and gliomas. Int J Cancer. 2000. 88:21–27.
Article40. Libby P. Atherosclerosis in Inflammation. Nature. 2002. 420:868–874.41. Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999. 340:115–126.42. Zhong H, May MJ, Jimi E, Ghosh S. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol Cell. 2002. 9:625–636.
Article43. Yang F, Tang E, Guan K, Wang CY. IKK beta plays an essential role in the phosphorylation of RelA/p65 on serine 536 induced by lipopolysaccharide. J Immunol. 2003. 170:5630–5635.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- LIGHT is Expressed in Foam Cells and Involved in Destabilization of Atherosclerotic Plaques through Induction of Matrix Metalloproteinase-9 and IL-8
- PMA-induced up-regulation of MMP-9 is regulated by a PKCalpha-NF-kappaB cascade in human lung epithelial cells
- LPS Increases 5-LO Expression on Monocytes via an Activation of Akt-Sp1/NF-kappaB Pathways
- Activation of Nuclear Factor-kappaB in the Atherosclerotic Human Coronary Artery
- Salicylate Regulates Cyclooxygenase-2 Expression through ERK and Subsequent NF-kappaB Activation in Osteoblasts